| The pseudoalkaloid diterpene Taxol® (paclitaxel) emerged as a best-selling anti-cancer drug in the mid-1990s. The compound attracted considerable interest because of its unique mechanism to stabilize microtubules, thus reducing dynamicity and ultimately promoting mitotic arrest. Taxol was originally isolated from members of the genus Taxus. Over the last 50 years, close to 600 metabolites with taxane scaffolds were isolated from various Taxus species and their structures reported. The Yew Genomics Resource provides an overview of the known chemical diversity of taxanes, with an emphasis on the functionalization of diterpene scaffolds. A more comprehensive assessment of the field is available in a recently published review article in Phytochemistry (LINK TO BE PROVIDED SOON).
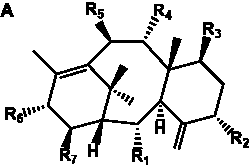
The term taxane is used for a large and diverse class of metabolites originally isolated from members of the genus Taxus (yews). Structures of taxanes can be classified as belonging to one of eleven types based on the arrangement of their fused ring systems (Table 1).
Table 1. Classification of taxane ring systems.
__________________________________________________________________________________________________________________
|
|
Type
|
Ring System |
Common Name |
Count
|
Skeleton with Carbon Chain Numbering |
|
__________________________________________________________________________________________________________________________________
|
|
|
|
|
|
|
|
I
|
6/8/6 |
Normal 6/8/6 taxanes |
349
|
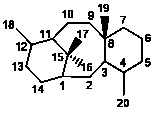 |
|
II
|
5/7/6 |
11(15→1)Abeotaxanes |
127
|
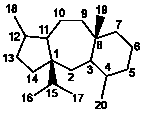 |
|
III
|
5/6/6 |
11(15→1),11(10→9)Diabeotaxanes |
12
|
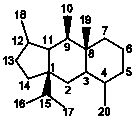 |
|
IV
|
6/10/6 |
2(3→20)Abeotaxanes |
35
|
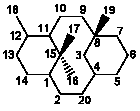 |
|
V
|
6/5/5/6 |
3,11-Cyclotaxanes |
26
|
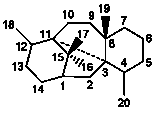 |
|
VI
|
6/8/6/6 |
14,20-Cyclotaxanes |
1
|
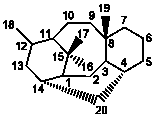 |
|
VII
|
6/5/5/5/6 |
3,11:12,20-Dicyclotaxanes |
4
|
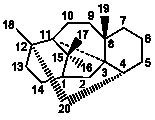 |
|
VIII
|
6/5/5/4/6 |
3,11:4,12-Dicyclotaxanes |
1
|
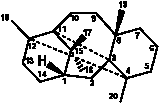 |
|
IX
|
5/5/4/6/6/6 |
3,11:4,12:14,20-Tricyclotaxanes |
1
|
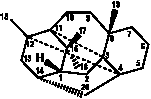 |
|
X
|
6/12 |
3,8-Secotaxanes |
35
|
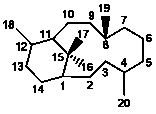 |
|
XI
|
8/6 |
11,12-Secotaxanes |
1
|
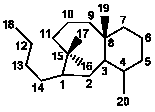 |
| _________________________________________________________________________________________________________________________________ |
|
The term taxane is used for a large and diverse class of metabolites originally isolated from members of the genus Taxus (yews). Taxoids are derivatives of Taxol and are therefore a smaller group within the taxane class, but the term is sometimes used synonymously with ‘taxanes’. A searchable listing of taxane structures isolated from members of the genus Taxus is provided in twentyfour tables below. Please note that, to ensure consistency throughout, we have adopted a unified nomenclature for taxanes that may differ from the trivial names given in the original papers. The Spektraris records mentioned in the tables below refer to a database of nuclear magnetic resonance (NMR) spectra of all known taxanes (plus roughly 21,000 other spectral records of plant natural products) (http://langelabtools.wsu.edu/nmr). The data tables are followed by an extensive list of references.
|
Table 2. Group I.1. 6/8/6-Taxanes. Neutral taxa-4(20),11-dienes.
__________________________________________________________________________________________________________________
A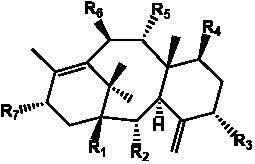 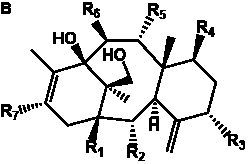 a a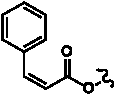 b b
| Abbreviations: |
| OAc, O-acetyl |
| OBz, O-benzoyl |
| OGlc, O-β-D-glucopyranosyl |
| |
| Symbols: |
| =O, ketone functional group |
|
| _________________________________________________________________________________________________________________ |
| |
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
R7 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-1 |
C-2 |
C-5 |
C-7 |
C-9 |
C-10 |
C-13 |
Record |
Source(s) |
|
| ______________________________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxa-4(20),11-dienes with hydroxyl functional groups |
| 5α,9α,10β,13α-Tetrahydroxytaxa- |
C20H32O4 |
336.2301 |
A |
H |
H |
OH |
H |
OH |
OH |
OH |
939 |
T. baccata |
Chan et al. (1966); |
| 4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxa-4(20),11-dienes with hydroxyl, O-acetyl, and/or O-glucosyl functional groups |
| 2-Deacetoxy-5-decinnamoyltaxinine J |
C26H38O9 |
494.2516 |
A |
H |
H |
OH |
H |
H |
OAc |
OAc |
69 |
T. yunnanensis; |
Chen et al. (1994a) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. baccata; |
Barboni et al. (1995a) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. wallichiana |
Viterbo et al. (1997) |
| 9α,10β-Diacetoxytaxa-4(20),11-diene- |
C24H36O6 |
420.2512 |
A |
H |
H |
OH |
H |
OAc |
OAc |
OH |
3259 |
T. baccata |
Della Casa de Mercano and Halsall |
| 5α,13α-diol |
|
|
|
|
|
|
|
|
|
|
|
|
(1969) |
| 10β,13α-Diacetoxy-taxa-4(20),11-diene |
C24H36O6 |
420.2512 |
A |
H |
H |
OH |
H |
OH |
OAc |
OAc |
3391 |
T. canadensis |
Zhang et al. (2012a) |
| 5α,9α-diol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 5α,13α-Diacetoxytaxa-4(20),11-diene- |
C24H36O6 |
420.2512 |
A |
H |
H |
OAc |
H |
OH |
OH |
OAc |
75 |
T. cuspidata |
Shi et al. (2001) |
| 9α,10β-diol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 9α,10β,13α-Triacetoxytaxa-4(20),11- |
C26H38O7 |
462.2618 |
A |
H |
H |
OH |
H |
OAc |
OAc |
OAc |
48 |
T. mairei |
Yang et al. (1996) |
| dien-5α-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxusin |
C28H40O8 |
504.2723 |
A |
H |
H |
OAc |
H |
OAc |
OAc |
OAc |
17 |
T. baccata |
Della Casa de Mercano and Halsall |
| |
|
|
|
|
|
|
|
|
|
|
|
|
(1969) |
| 9α,10β-Diacetoxy-5α-(β-D-glucopyranosyl) |
C30H46O11 |
582.3040 |
A |
H |
H |
OGlc |
H |
OAc |
OAc |
OH |
63 |
T. canadensis |
Shi et al. (2003a) |
| taxa-4(20),11-dien-13α-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Decinnamoyl-1-hydroxytaxinine J |
C30H42O12 |
594.2676 |
A |
H |
H |
OH |
OAc |
OAc |
OAc |
OAc |
70 |
T. baccata |
Barboni et al. (1995a) |
| 7β-Acetoxytaxusin |
C30H42O10 |
562.2778 |
A |
H |
H |
OAc |
OAc |
OAc |
OAc |
OAc |
3362 |
T. baccata |
Della Casa de Mercano and Halsall |
| (5α,7β,9α,10β,13α-pentaacetoxytaxa- |
|
|
|
|
|
|
|
|
|
|
|
|
(1969) |
| 4(20),11-diene) |
|
|
|
|
|
|
|
|
|
|
|
T. globosa |
Guerrero et al. (2000) |
| 7β,9α,10β-Triacetoxy-13α-hydroxy-5α-O-β- |
C32H48O13 |
640.3095 |
A |
H |
H |
OGlc |
OAc |
OAc |
OAc |
OH |
3390 |
T. mairei |
Sun et al. (2015) |
| (D-glucopyranosyl)taxa-4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10β,13α-Diacetoxytaxa-4(20),11-diene- |
C24H36O7 |
436.2461 |
A |
H |
OH |
OH |
H |
OH |
OAc |
OAc |
47 |
T. chinensis |
Zhang et al. (1995a) |
| 2α,5α,9α-triol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,9α,10β-Triacetoxytaxa-4(20),11- |
C26H38O8 |
478.2567 |
A |
H |
OAc |
OH |
H |
OAc |
OAc |
OH |
937 |
T. mairei |
Shi et al. (2000b) |
| diene-5α,13α-diol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,9α,13α-Triacetoxytaxa-4(20),11- |
C26H38O8 |
478.2567 |
A |
H |
OAc |
OH |
H |
OAc |
OH |
OAc |
56 |
T. canadensis |
Shi et al. (2003b) |
| diene-5α,10β-diol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,10β,13α-Triacetoxytaxa-4(20),11- |
C26H38O8 |
478.2567 |
A |
H |
OAc |
OH |
H |
OH |
OAc |
OAc |
54 |
T. canadensis |
Shi et al. (2003b) |
| diene-5α,9α-diol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Decinnamoyltaxinine E |
C28H40O9 |
520.2672 |
A |
H |
OAc |
OH |
H |
OAc |
OAc |
OAc |
46 |
T. canadensis |
Chen et al. (1999) |
| 2α,9α,10β-Triacetoxy-13α-(β-D-gluco- |
C32H48O13 |
640.3095 |
A |
H |
OAc |
OH |
H |
OAc |
OAc |
OGlu |
3885 |
T. cuspidata |
Ni et al. (2011) |
| pyranosyloxy)taxa-4(20),11-dien-5α-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10β-Acetoxy-taxa-4(20),11-diene- |
C22H34O7
|
410.2305 |
A |
H |
OH |
OH |
OH |
OH |
OAc |
OH |
64 |
T. cuspidata |
Huo et al. (2007a) |
| 2α,5α,7β,9α,13α-pentaol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,5α,9α,10β,13α-Pentaacetoxytaxa- |
C30H42O10 |
562.2778 |
A |
H |
OAc |
OAc |
H |
OAc |
OAc |
OAc |
3314 |
T. baccata |
Della Casa de Mercano and Halsall |
| 4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
(1969) |
| 2-Deacetyl-5-decinnamoyltaxinine E |
C26H38O8 |
478.2567 |
A |
H |
OH |
OH |
OAc |
OAc |
OAc |
OAc |
68 |
T. baccata |
Barboni et al. (1995a) |
| 7,9-Deacetyl-5-decinnamoyltaxinine J |
C26H38O9 |
494.2516 |
A |
H |
OAc |
OH |
OH |
OH |
OAc |
OAc |
45 |
T. cuspidata |
Zamir et al. (1999a) |
| Taxezopidine F |
C28H40O10 |
536.2621 |
A |
H |
OAc |
OH |
OAc |
OAc |
OAc |
OH |
785 |
T. cuspidata |
Wang et al. (1998) |
| Taxumariene D |
C28H40O10 |
536.2622 |
A |
H |
OAc |
OH |
OH |
OAc |
OAc |
OAc |
3475 |
T. mairei |
Chen et al. (2020) |
| Taxumariene E |
C28H40O10 |
536.2622 |
A |
H |
OAc |
OH |
OAc |
OH |
OAc |
OAc |
3476 |
T. mairei |
Chen et al. (2020) |
| Decinnamoyltaxinine J |
C33H42O12 |
630.2676 |
A |
H |
OAc |
OH |
OAc |
OAc |
OAc |
OAc |
933 |
T. brevifolia |
Kingston et al. (1982) |
| 2α,5α,7β,9α,10β,13α-Hexaacetoxy- |
C32H44O12 |
620.2833 |
A |
H |
OAc |
OAc |
OAc |
OAc |
OAc |
OAc |
19 |
T. chinensis |
Della Casa de Mercano and Halsall |
| taxa-4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
(1969) |
| 1-Hydroxy-2-deacetoxy-5-decinnamoyl- |
C28H40O10 |
536.2621 |
A |
OH |
H |
OH |
OAc |
OAc |
OAc |
OAc |
51 |
T. wallichiana |
Chattopadhyay et al. (2006) |
| taxinine J |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxawallin G |
C26H38O9 |
494.2516 |
A |
OH |
H |
OAc |
OH |
OH |
OAc |
OAc |
76 |
T. wallichiana |
Zhang et al. (1995a) |
| 1β,7β,9α,10β,-Tetraacetoxytaxa-4(20),11- |
C28H40O9 |
520.2672 |
A |
OAc |
H |
OH |
OAc |
OAc |
OAc |
H |
66 |
T. baccata |
Topcu et al. (1994) |
| dien-5α-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,7β,9α,13α-Tetraacetoxy-5α,10β,17- |
C28H40O11 |
552.2571 |
B |
H |
OAc |
OH |
OAc |
OAc |
OH |
OAc |
4407 |
T. mairei |
Zhou et al. (2014) |
| trihydroxytaxa-4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxa-4(20),11-dienes with benzoyl or cinnamoyl functional groups |
| 2α-Benzoyloxy-9α,10β,13α-triacetoxy- |
C33H42O10 |
598.2778 |
A |
OH |
OBz |
OH |
H |
OAc |
OAc |
OAc |
72 |
T. chinensis |
Jia and Zhang (1991) |
| taxa-4(20),11-diene-1β,5α-diol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 13-Acetylbrevifoliol |
C33H42O10 |
598.2778 |
A |
OH |
H |
OH |
OBz |
OAc |
OAc |
OAc |
50 |
T. wallichiana |
Barboni et al. (1993) |
| 2α,5α,9α,10β-Tetraacetoxy-13α-(Z)- |
C37H46O10 |
650.3091 |
A |
H |
OAc |
OAc |
H |
OAc |
OAc |
a |
65 |
T. cuspidata |
Shi et al. (2003c) |
| cinnamoyltaxa-4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxa-4(20),11-dienes with a keto functional group |
| Taxuyunnanine D |
C24H34O5 |
402.2406 |
A |
H |
H |
OAc |
H |
H |
=O |
OAc |
929 |
T. yunnanensis |
Zhang et al. (1994a) |
| Taxezopidine C |
C22H32O6 |
392.2199 |
A |
H |
OH |
H |
H |
OH |
OH |
=O |
58 |
T. cuspidata |
Wang et al. (1998) |
| Taxezopidine D |
C22H32O6 |
392.2199 |
A |
H |
OAc |
H |
H |
OH |
OAc |
=O |
59 |
T. cuspidata |
Wang et al. (1998) |
| Taxuspine G (2-deacetyltaxinine A) |
C24H34O7 |
434.2305 |
A |
H |
OH |
OH |
H |
OAc |
OAc |
=O |
60 |
T. cuspidata |
Kobayashi et al. (1995a) |
| Taxinine A |
C26H36O8 |
476.2410 |
A |
H |
OAc |
OH |
H |
OAc |
OH |
=O |
3388 |
T. cuspidata |
Chiang et al. (1967) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. mairei |
Li and Shi (2000); Li et al. (2005b) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. chinensis |
Chiang et al. (1975) |
| 2α,10β-Diacetoxy-5α,9α-dihydoxytaxa- |
C24H34O7 |
434.2305 |
A |
H |
OAc |
OH |
H |
OH |
OAc |
=O |
52 |
T. canadensis |
Shi et al. (2003b) |
| 4(20),11-dien-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxuspinanane K |
C26H36O8 |
476.2410 |
A |
H |
H |
OH |
OAc |
OAc |
OAc |
=O |
62 |
T. cuspidata |
Morita et al. (1998) |
| Taxinine H |
C29H38O9 |
530.2516 |
A |
H |
OAc |
OAc |
H |
OAc |
OAc |
=O |
880 |
T. cuspidata |
Chiang et al. (1967) |
| 2α,9α,10β-Triacetoxy-5α-(β-D-gluco- |
C32H46O13 |
638.2938 |
A |
H |
OAc |
OGlc |
H |
OAc |
OAc |
=O |
57 |
T. cuspidata |
Li et al. (2005a) |
| pyranoside)taxa-4(20),11-dien-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10β-Acetoxy-2α,5α,7β,9α-tetrahydroxy- |
C22H32O7 |
408.2148 |
A |
H |
OH |
OH |
OH |
OH |
OAc |
=O |
56 |
T. yunnanensis |
Nguyen et al. (2003) |
| taxa-4(20),11-dien-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,9α-Diacetoxy-5α,10β-dihydroxytaxa- |
C24H34O7 |
434.2305 |
A |
H |
OAc |
OH |
H |
OAc |
OH |
=O |
52 |
T. canadensis |
Shi et al. (2003b) |
| 4(20),11-dien-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2,10-Diacetoxy-5-decinnamoyltaxicin I |
C24H34O8 |
450.2254 |
A |
OH |
OAc |
OH |
H |
OH |
OAc |
=O |
71 |
T. baccata |
Barboni et al. (1995a) |
| 1β-Hydroxytaxinine A |
C26H36O9 |
492.2359 |
A |
OH |
OAc |
OH |
H |
OAc |
OAc |
=O |
67 |
T. baccata |
Barboni et al. (1995a); |
| 2α,7β,9α-Triacetoxy-5α,10β-dihydroxytaxa- |
C26H36O9 |
492.2359 |
A |
H |
OAc |
OH |
OAc |
OAc |
OH |
=O |
3472 |
T. cuspidata |
Li et al. (2013) |
| 4(20),11-dien-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxuspine F |
C28H38O10 |
534.2465 |
A |
H |
OAc |
OH |
OAc |
OAc |
OAc |
=O |
61 |
T. cuspidata |
Kobayashi et al. (1995a) |
| 2α,7β,9α,13α-Tetraacetoxy-5α,10β,17- |
C28H40O11 |
552.2571 |
B |
H |
OAc |
OH |
OAc |
OAc |
OH |
OAc |
4407 |
T. mairei |
Zhou et al. (2014) |
| trihydroxy-taxa-4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxa-4(20),11-dienes for which corrected structures have been proposed |
| 2α,5α,7β,9α,10β,13α-Hexahydroxytaxa- |
C20H32O6 |
368.2199 |
A |
H |
OH |
OH |
OH |
OH |
OH |
OH |
n.a. |
T. chinensis |
Jia and Zhang (1991); |
| 4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
corrected by Huang et al. (1998) |
| 7-Debenzoyloxy-10-deacetylbrevifoliol |
C22H34O6 |
394.2355 |
A |
OH |
H |
OH |
H |
OAc |
OH |
OH |
n.a. |
T. wallichiana |
Barboni et al. (1993) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
corrected by Appendino et al.
(1993b) |
| Brevifoliol |
C31H40O9 |
556.2672 |
A |
OH |
H |
OH |
OAc |
OAc |
OBz |
OH |
n.a. |
T. brevifolia |
Balza et al. (1991); Chu et al.
(1993a); |
| |
|
|
|
|
|
|
|
|
|
|
|
|
corrected by Appendino et al.
(1993b) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
and Chu et al. (1994) |
| 2α-Acetoxybrevifoliol |
C33H42O11 |
614.2727 |
A |
OH |
OAc |
OH |
OAc |
OAc |
OBz |
OH |
n.a. |
T. baccata |
Appendino et al. (1993a) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
corrected by Appendino et al.
(1993b) |
| 2α-Benzoyl-9α,10β-diacetoxy-taxa- |
C31H40O9 |
556.2672 |
A |
OH |
OBz |
OH |
H |
OAc |
OAc |
OH |
n.a. |
T. chinensis |
Jia and Zhang (1991) |
| taxa-4(20),11-diene-1β,5α,13α-triol |
|
|
|
|
|
|
|
|
|
|
|
|
corrected by Huang et al. (1998) |
| 5α,7β,10β-Triacetoxy-2α-(α-methylbutyryl- |
C31H46O8 |
546.3193 |
A |
H |
b |
OAc |
OAc |
H |
OAc |
H |
n.a. |
T. baccata |
Della Casa de Mercano and Halsall |
| oxy)taxa-4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
(1969); |
| |
|
|
|
|
|
|
|
|
|
|
|
|
corrected by Topcu et al. (1994) |
| 2α-(α-Methylbutyryloxy)-7β,9α,10β-tri- |
C31H46O9 |
562.3142 |
A |
H |
b |
OH |
OAc |
OAc |
OAc |
H |
n.a. |
T. baccata |
Della Casa de Mercano and Halsall |
| acetoxytaxa-4(20),11-dien-5α-ol |
|
|
|
|
|
|
|
|
|
|
|
|
(1969); |
| 2α-(α-Methylbutyryloxy)-5α,7β,9α,10β- |
C33H48O10 |
604.3247 |
A |
H |
b |
OAc |
OAc |
OAc |
OAc |
H |
n.a. |
T. baccata |
Della Casa de Mercano and Halsall |
| tetraacetoxytaxa-4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
(1969); |
| |
|
|
|
|
|
|
|
|
|
|
|
|
corrected by Gabetta et al.
(1995a) |
| ________________________________________________________________________________________________________________ |
Table 3. Group I.2. 6/8/6-Taxanes. Taxa-4(20),11-dienes with C-5 cinnamoyl side chain.
________________________________________________________________________________________________________________
A 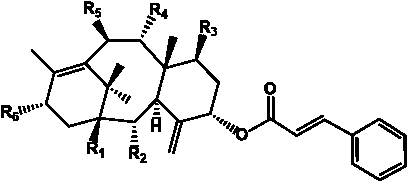
| Abbreviations: |
| OAc, O-acetyl |
| OBz, O-benzoyl |
| |
| Symbols: |
| =O, ketone functional group |
| ________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-1 |
C-2 |
C-7 |
C-9 |
C-10 |
C-13 |
Record |
Source(s) |
|
| ___________________________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| C-5 Cinnamoyltaxadienes with hydroxyl, acetyl and/or benzoyl functional groups |
| Dantaxusin B |
C33H42O7 |
550.2931 |
A |
H |
H |
H |
OH |
OAc |
OAc |
93 |
T. yunnanensis |
Shinozaki et al. (2001) |
| 9α,10β-Diacetoxy-5α-cinnamoyloxytaxa- |
C33H42O7 |
550.2931 |
A |
H |
H |
H |
OAc |
OAc |
OH |
101 |
T. yunnanensis |
Shi et al. (2000b) |
| 4(20),11-dien-13α-ol |
|
|
|
|
|
|
|
|
|
|
T. cuspidata |
Shi et al. (2000b) |
| 5α-Cinnamoyloxy-9α,10β,13α-triacetoxy- |
C35H44O8 |
592.3036 |
A |
H |
H |
H |
OAc |
OAc |
OAc |
94 |
T. mairei |
Yeh et al. (1988) |
| taxa-4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
| 2-Deacetoxy-7,9-dideacetyltaxinine J |
C33H42O8 |
566.2880 |
A |
H |
H |
OH |
OH |
OAc |
OAc |
943 |
T. chinensis |
Liang et al. (1998) |
| 9α,10β,13α-Triacetoxy-5α-cinnamoyloxy- |
C35H44O9 |
608.2985 |
A |
H |
H |
OH |
OAc |
OAc |
OAc |
95 |
T. mairei |
Yang et al. (1996) |
| taxa-4(20),11-dien-7β-ol |
|
|
|
|
|
|
|
|
|
|
|
|
| Taxezopidine H |
C35H44O9 |
608.2985 |
A |
H |
H |
OAc |
OAc |
OAc |
OH |
100 |
T. cuspidata |
Wang et al. (1998) |
| 2-Deacetoxytaxinine J |
C37H46O10 |
650.3091 |
A |
H |
H |
OAc |
OAc |
OAc |
OAc |
1005 |
T. mairei |
Liang et al. (1988) |
| |
|
|
|
|
|
|
|
|
|
|
T. cuspidata |
Mao et al. (1999) |
| 9α,10β-Diacetoxy-5α-cinnamoyloxytaxa- |
C33H42O8 |
566.2880 |
A |
H |
OH |
H |
OAc |
OAc |
OH |
944 |
T. chinensis |
Zhang and Jia (1991) |
| 4(20),11-diene-2α,13α-diol |
|
|
|
|
|
|
|
|
|
|
|
|
| Taxezopidine G |
C35H44O9 |
608.2985 |
A |
H |
OH |
H |
OAc |
OAc |
OAc |
96 |
T. cuspidata |
Wang et al. (1998) |
| |
|
|
|
|
|
|
|
|
|
|
T. mairei |
Li and Shi (1999) |
| Taxuspinanane G |
C37H46O11 |
666.3040 |
A |
H |
OH |
OAc |
OAc |
OAc |
OAc |
945 |
T. cuspidata |
Morita et al. (1998b) |
| |
|
|
|
|
|
|
|
|
|
|
T. baccata |
Verdian-Rizi et al. (2008) |
| 2α,10β-Diacetoxy-5α-cinnamoyloxytaxa- |
C33H40O8 |
564.2723 |
A |
H |
OAc |
H |
OH |
OAc |
OH |
99 |
T. canadensis |
Shi et al. (2003d) |
| 4(20),11-diene-9α,13α-diol |
|
|
|
|
|
|
|
|
|
|
|
|
| 9-Deacetyltaxinine E |
C35H44O9 |
608.2985 |
A |
H |
OAc |
H |
OH |
OAc |
OAc |
91 |
T. canadensis |
Zhang et al. (2000a) |
| |
|
|
|
|
|
|
|
|
|
|
T. mairei |
Li et al. (2005b) |
| 2α,9α,13α-Triacetoxy-5α-cinnamoyloxy- |
C35H48O9 |
612.3298 |
A |
H |
OAc |
H |
OAc |
OH |
OAc |
97 |
T. chinensis |
Zhang and Jia (1991) |
| taxa-4(20),11-dien-10β-ol |
|
|
|
|
|
|
|
|
|
|
|
|
| 13-Deacetyltaxinine E |
C35H44O9 |
608.2985 |
A |
H |
OAc |
H |
OAc |
OAc |
OH |
901 |
T. cuspidata |
Shi et al. (2000) |
| Taxinine E |
C37H46O10 |
650.3091 |
A |
H |
OAc |
H |
OAc |
OAc |
OAc |
3123 |
T. mairei |
Yeh et al. (1988) |
| Dantaxusin D |
C37H46O11 |
666.3040 |
A |
H |
OAc |
OH |
OAc |
OAc |
OAc |
92 |
T. yunnanensis |
Shinozaki et al. (2002) |
| Taxinine J |
C39H48O12 |
708.3146 |
A |
H |
OAc |
OAc |
OAc |
OAc |
OAc |
3162 |
T. mairei |
Min et al. (1989);
Liang et al. (1988) |
| Taxawallin A |
C37H46O11 |
666.3040 |
A |
OH |
H |
OAc |
OAc |
OAc |
OAc |
98 |
T. wallichiana |
Zhang et al. (1995c) |
| Taxusumatrin |
C39H48O13 |
724.3095 |
A |
OH |
OAc |
OAc |
OAc |
OAc |
OAc |
3477 |
T. sumatrana |
Kuo et al. (2015) |
| C-5 Cinnamoyltaxadienes with a C-13 ketone functional groups |
| 2-Deacetoxytaxinine B |
C35H42O9 |
606.2829 |
A |
H |
H |
OAc |
OAc |
OAc |
=O |
87 |
T. wallichiana |
Shrestha et al. (1997) |
| |
|
|
|
|
|
|
|
|
|
|
T. chinensis |
Liang et al. (1998) |
| 5α-Cinnamoyloxy-2α,9α,10β-trihydroxy- |
C29H36O6 |
480.2512 |
A |
H |
OH |
H |
OH |
OH |
=O |
77 |
T. baccata |
Shi et al. (2004a) |
| taxa-4(20),11-dien-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 5-Cinnamoyl-10-acetyltaxicin II |
C31H38O7 |
522.2618 |
A |
H |
OH |
H |
OH |
OAc |
=O |
83 |
T. baccata |
Appendino et al. (1992a) |
| 9α-Acetoxy-5α-cinnamoyloxytaxa-2α, |
C31H38O7 |
522.2618 |
A |
H |
OH |
H |
OAc |
OH |
=O |
78 |
T. baccata |
Shi et al. (2004a) |
| 10β-dihydroxy-4(20),11-dien-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 2-Deacetyltaxinine |
C33H40O8 |
564.2723 |
A |
H |
OH |
H |
OAc |
OAc |
=O |
3163 |
T. mairei |
Li et a. (2005);
Shi et al. (1999a) |
| 9,10-Deacetyltaxinine |
C31H38O7 |
522.2618 |
A |
H |
OAc |
H |
OH |
OH |
=O |
941 |
T. yunnanensis |
Shi et al. (1999a) |
| 9-Deacetyltaxinine |
C33H40O8 |
564.2723 |
A |
H |
OAc |
H |
OH |
OAc |
=O |
88 |
T. cuspidata |
Sakai et al. (2001) |
| 10-Deacetyltaxinine |
C33H40O8 |
564.2723 |
A |
H |
OAc |
H |
OAc |
OH |
=O |
80 |
T. mairei |
Shi et al. (2006a) |
| Taxinine |
C35H42O9 |
606.2829 |
A |
H |
OAc |
H |
OAc |
OAc |
=O |
3164 |
T. baccata |
Kurono et al. (1963) |
| |
|
|
|
|
|
|
|
|
|
|
T. chinensis |
Chiang et al. (1975) |
| |
|
|
|
|
|
|
|
|
|
|
T. cuspidata |
Chiang et al. (1967) |
| |
|
|
|
|
|
|
|
|
|
|
T. mairei |
Liu et al. (1984) |
| Taxezopidine E |
C31H38O8 |
538.2567 |
A |
H |
OH |
OH |
OH |
OAc |
=O |
85 |
T. cuspidata |
Wang et al. (1998) |
| 7,9-Deacetyltaxinine B |
C33H40O9 |
580.2672 |
A |
H |
OAc |
OH |
OH |
OAc |
=O |
79 |
T. canadensis |
Zamir et al. (1999b) |
| 9-Deacetyltaxinine B |
C35H42O10 |
622.2778 |
A |
H |
OAc |
OAc |
OH |
OAc |
=O |
89 |
T. mairei |
Shi et al. (2000a) |
| 10-Deacetyltaxinine B |
C35H42O10 |
622.2778 |
A |
H |
OAc |
OAc |
OAc |
OH |
=O |
90 |
T. cuspidata |
Tong et al. (1993) |
| Taxinine B |
C37H44O11 |
664.2884 |
A |
H |
OAc |
OAc |
OAc |
OAc |
=O |
3166 |
T. cuspidata |
Woods et al. (1968) |
| 5α-Cinnamoyloxy-1β,2α,9α,10β-tetra- |
C29H36O7 |
496.2461 |
A |
OH |
OH |
H |
OH |
OH |
=O |
74 |
T. baccata |
Shi et al. (2004a) |
| hydroxytaxa-4(20),11-dien-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 5-cinnamoyl-10-acetyltaxicin I |
C31H38O8 |
538.2567 |
A |
OH |
OH |
H |
OH |
OAc |
=O |
84 |
T. baccata |
Appendino et al. (1992a) |
| O-Cinnamoyltaxicin I |
C29H36O7 |
496.2461 |
A |
OH |
OH |
H |
OAc |
OH |
=O |
905 |
T. baccata |
Baxter et al. (1962) |
| 5-Cinnamoyl-9,10-diacetyltaxicin I |
C33H40O9 |
580.2672 |
A |
OH |
OH |
H |
OAc |
OAc |
=O |
86 |
T. baccata |
Appendino et al. (1994a) |
| 2-O-Acetyl-5-O-cinnamoyltaxicin I |
C31H38O8 |
538.2567 |
A |
OH |
OAc |
H |
OH |
OH |
=O |
894 |
T. baccata |
Appendino et al. (1992a) |
| O-Cinnamoyltaxicin I triacetate |
C35H42O10 |
622.2778 |
A |
OH |
OAc |
H |
OAc |
OAc |
=O |
3167 |
T. baccata |
Baxter et al. (1962) |
| 1β,7β-Dihydroxytaxinine |
C35H42O11 |
638.2727 |
A |
OH |
OAc |
OH |
OAc |
OAc |
=O |
82 |
T. cuspidata |
Cheng et al. (2000) |
| 1β-hydroxy-7β-acetoxytaxinine |
C37H44O12 |
680.2833 |
A |
OH |
OAc |
OAc |
OAc |
OAc |
=O |
81 |
T. cuspidata |
Cheng et al. (2000) |
| C-5 Cinnamoyltaxadienes for which corrected structures have been proposed |
| 9α,10β-Diacetoxy-2α-benzoyloxy-5α- |
C40H46O10 |
686.3091 |
A |
OH |
OBz |
H |
OAc |
OAc |
OH |
3225 |
T. chinensis |
Jia and Zhang (1991); |
| cinnamoyloxytaxa-4(20),11-diene- |
|
|
|
|
|
|
|
|
|
|
T. chinensis |
Zhang et al. (1991);
Huang et al. (1998) |
| 1β,13α-diol |
|
|
|
|
|
|
|
|
|
|
|
|
| ________________________________________________________________________________________________________________ |
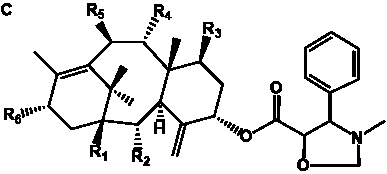
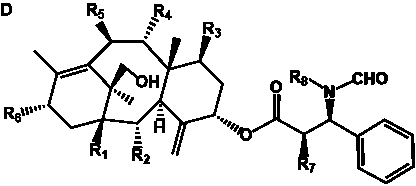
Table 4. Group I. 3. 6/8/6-Taxanes. Taxa-4(20),11-dienes with a basic C-5 side chain.
________________________________________________________________________________________________________________
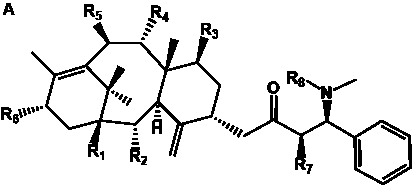
| Abbreviations: |
| OAc, O-acetyl |
| |
| Symbols: |
| =O, ketone functional group |
| _________________________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
R7 |
R8 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-1 |
C-2 |
C-7 |
C-9 |
C-10 |
C-13 |
C-2' |
N-3' |
Record |
Source(s) |
|
| _________________________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxa-4(20),11-dienes with a basic C-5 side chain and hydroxyl and/or acetyl functional groups |
| 9α,10β,13α-Triacetoxy-5α-[(R)-3'-methyl- |
C36H49NO8 |
623.3458 |
A |
H |
H |
H |
OAc |
OAc |
OAc |
H |
H |
952 |
T. mairei |
Shi et al. (1999c) |
| amino-3'-phenylpropanoyloxy] taxa- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 7,2'-Didesacetoxyaustrospicatine |
C37H51NO8 |
637.3615 |
A |
H |
H |
H |
OAc |
OAc |
OAc |
H |
CH3 |
3511 |
T. wallichiana |
Zhang et al. (1995c) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
T. mairei |
Li and Shi (2000) |
| 7β,9α,10β,13α-Tetraacetoxy-5α-[(R)-3'- |
C38H51NO10 |
681.3513 |
A |
H |
H |
OAc |
OAc |
OAc |
OAc |
H |
H |
3553 |
T. mairei |
Shi et al. (1999d) |
| methylamino-3'-phenylpropanoyloxy] |
|
|
|
|
|
|
|
|
|
|
|
|
T. cuspidata |
Ishiyama et al. (2007) |
| taxa-4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2'β-Deacetoxyaustrospicatine |
C39H53NO10 |
695.3669 |
A |
H |
H |
OAc |
OAc |
OAc |
OAc |
H |
CH3 |
113 |
T. wallichiana |
Chattopadhyay et al. (1996) |
| 10β,13α-Diacetoxy-5α-[(R)-3'-methyl- |
C34H47NO8 |
597.3302 |
A |
H |
OH |
H |
OH |
OAc |
OAc |
H |
H |
115 |
T. baccata |
Appendino et al. (1993c) |
| amino-3'-phenylpropanoyloxy] taxa- |
|
|
|
|
|
|
|
|
|
|
|
|
T. canadensis |
Shi et al. (2004d) |
| 4(20),11-diene-2α,9α-diol |
|
|
|
|
|
|
|
|
|
|
|
|
T. baccata |
Appendino et al. (1993c) |
| Taxezopidine O |
C36H49NO9 |
639.3407 |
A |
H |
OH |
H |
OAc |
OAc |
OAc |
H |
H |
953 |
T. cuspidata |
Ishiyama et al. (2007) |
| 13-Deoxo-13α-acetyloxy-1-deoxytaxine B |
C35H49NO8 |
611.3458 |
A |
H |
OH |
H |
OH |
OAc |
OAc |
H |
CH3 |
109 |
T. baccata |
Appendino et al. (1993c) |
| Taxuspine Z |
C37H51NO9 |
653.3564 |
A |
H |
OH |
H |
OAc |
OAc |
OAc |
H |
CH3 |
111 |
T. cuspidata |
Shigemori et al. (1997) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
T. chinensis |
Chen et al. (1999) |
| 2α,10β,13α-Triacetoxy-5α-[(R)-3'-methyl- |
C36H49NO9 |
639.3407 |
A |
H |
OAc |
H |
OH |
OAc |
OAc |
H |
H |
116 |
T. canadensis |
Shi et al. (2003b) |
| amino-3'-phenylpropanoyloxy] taxa- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 4(20),11-dien-9α-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,9α,10β,13α-Tetraacetoxy-5α-[(R)-3'- |
C38H51NO10 |
681.3513 |
A |
H |
OAc |
H |
OAc |
OAc |
OAc |
H |
H |
3568 |
T. mairei |
Shi et al. (1999d) |
| methylamino-3'-phenylpropanoyloxy] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| taxa-4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,9α,13α-Triacetoxy-5α-[(R)-3'-dimethyl- |
C37H51NO9 |
653.3564 |
A |
H |
OAc |
H |
OAc |
OH |
OAc |
H |
CH3 |
3571 |
T. canadensis |
Shi et al. (2003a) |
| amino-3'-phenylpropanoyloxy] taxa- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 4(20),11-dien-10β-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,10β,13α-Triacetoxy-5α-[(R)-3'-dimethyl- |
C37H51NO9 |
653.3564 |
A |
H |
OAc |
H |
OH |
OAc |
OAc |
H |
CH3 |
3573 |
T. canadensis |
Shi et al. (2003a) |
| amino-3'-phenylpropanoyloxy] taxa- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 4(20),11-dien-9α-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α-Acetoxy-2',7-dideacetoxyaustro- |
C39H53NO10 |
695.3669 |
A |
H |
OAc |
H |
OAc |
OAc |
OAc |
H |
CH3 |
112 |
T. chinensis |
Chen et al. (1999) |
| spicatine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 7β,9α,10β,13α-Tetraacetoxy-5α-[(2'R- |
C39H53NO12 |
727.3568 |
A |
H |
OH |
OAc |
OAc |
OAc |
OAc |
OH |
CH3 |
108 |
T. canadensis |
Shi et al. (2004b) |
| 3'S)-N,N- dimethyl-3'-phenylisoseryl- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| oxy]taxa-4(20),11-dien-2α-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α-Acetoxy-2'β-deacetyl-austrospicatine |
C41H55NO13 |
769.3673 |
A |
H |
OAc |
OAc |
OAc |
OAc |
OAc |
OH |
CH3 |
3575 |
T. wallichiana |
Prasain et al. (2001) |
| 9α,13α-Diacetoxy-5α-[(R)-3'-dimethyl- |
C35H49NO8 |
611.3458 |
A |
OH |
H |
H |
OAc |
OH |
OAc |
H |
CH3 |
949 |
T. mairei |
Shi et al. (1999b) |
| amino-3'-phenylpropanoyloxy] taxa- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 4(20),11-dien-1β,10β-diol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 13-Deoxo-13α-acetyloxytaxine B |
C35H49NO9 |
627.3407 |
A |
OH |
OH |
H |
OH |
OAc |
OAc |
H |
CH3 |
110 |
T. baccata |
Appendino et al. (1993c) |
| (+)-2α-Acetoxy-2',7-dideacetoxy-1- |
C39H53NO11 |
711.3619 |
A |
OH |
OAc |
H |
OAc |
OAc |
OAc |
H |
CH3 |
114 |
T. baccata |
Doss et al. (1997) |
| hydroxyaustrospicatine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α-Acetoxy-2'-deacetyl-1-hydroxy- |
C41H55NO14 |
785.3623 |
A |
OH |
OAc |
OAc |
OAc |
OAc |
OAc |
OH |
CH3 |
932 |
T. baccata |
Barboni et al. (1995a) |
| austrospicatine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 9α,10β,13α-Triacetoxy-5α-[(R)-3'-dimethyl- |
C37H51NO10 |
669.3513 |
B |
H |
OH |
H |
OAc |
OAc |
OAc |
H |
CH3 |
117 |
T. canadensis |
Shi et al. (2004b) |
| amino-3'-phenylpropanoyloxy]taxa- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 4(20),11-diene-2α,17-diol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxine NA-13 |
C37H47NO10 |
665.3200 |
C |
H |
OAc |
H |
OAc |
OAc |
OAc |
n.a. |
n.a. |
955 |
T. cuspidata |
Wang et al. (2008) |
| 7β,9α,10β,13α-Tetraacetoxy-5α-[3'-(N- |
C39H51NO11 |
709.3462 |
D |
H |
H |
OAc |
OAc |
OAc |
OAc |
H |
CH3 |
3553 |
T. canadensis |
Zhang et al. (2008a) |
| formyl-N-methylamino)-3'-phenyl- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| propanoyloxy]taxa-4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxa-4(20),11-dienes with a basic C-5 side chain and a C-13 ketone functional group |
| 10β-Acetoxy-5α-[(3'-dimethylamino-3'- |
C34H45NO6 |
563.32469 |
A |
H |
H |
H |
OH |
OAc |
=O |
H |
CH3 |
3873 |
T. cuspidata |
Wang et al. (2013) |
| phenyl)-propionyloxy]moiety-9α-hydroxy- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| taxa-4(20)11-dien-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2-Deacetoxy-10-acetoxytaxine B |
C33H45NO7 |
567.3196 |
A |
H |
OH |
H |
OH |
OAc |
=O |
H |
CH3 |
103 |
T. baccata |
Jenniskens et al. (1996) |
| 2-Deacetoxy-9-acetoxytaxine B |
C33H45NO7 |
567.3196 |
A |
H |
OH |
H |
OAc |
OH |
=O |
H |
CH3 |
102 |
T. baccata |
Jenniskens et al. (1996) |
| 2-Deacetyltaxine II |
C35H47NO8 |
609.3302 |
A |
H |
OH |
H |
OAc |
OAc |
=O |
H |
CH3 |
946 |
T. cuspidata |
Shi et al. (2000d) |
| 1-Deoxy-2α-acetoxytaxine B |
C35H47NO8 |
609.3302 |
A |
H |
OAc |
H |
OH |
OAc |
=O |
H |
CH3 |
947 |
T. cuspidata |
Shi et al. (2000d) |
| 1-Deoxy-2α,9α-diacetoxy-10-deacetyl- |
C35H48NO8 |
610.3380 |
A |
H |
OAc |
H |
OAc |
OH |
=O |
H |
CH3 |
948 |
T. cuspidata |
Shi et al. (2000d) |
| taxine B |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2'-Hydroxytaxine II |
C37H49NO10 |
667.3356 |
A |
H |
OAc |
H |
OAc |
OAc |
=O |
OH |
CH3 |
107 |
T. cuspidata |
Ando et al. (1997) |
| 2α,7β,9α,10β-Tetraacetoxy-5α-[(2'R,3'S)- |
C39H51NO12 |
725.3411 |
A |
H |
OAc |
OAc |
OAc |
OAc |
=O |
OH |
CH3 |
902 |
T. cuspidata |
Shi et al. (2000c) |
| N,N-dimethyl-3'-phenylisoseryloxy] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| taxa-4(20),11-dien-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxine B |
C33H45NO8 |
583.3145 |
A |
OH |
OH |
H |
OH |
OAc |
=O |
H |
CH3 |
104 |
T. baccata |
Ettouati et al. (1991) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
(first reported by Graf et al. |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
(1986) with incorr. structure) |
| 10-Acetoxytaxine B |
C35H47NO9 |
625.3251 |
A |
OH |
OAc |
H |
OH |
OAc |
=O |
H |
CH3 |
105 |
T. baccata |
Jenniskens et al. (1996) |
| 9-Acetoxytaxine B |
C35H47NO9 |
625.3251 |
A |
OH |
OAc |
H |
OAc |
OH |
=O |
H |
CH3 |
106 |
T. baccata |
Jenniskens et al. (1996) |
| ____________________________________________________________________________________________________________________________________________________________________________________ |
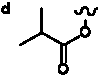
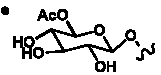
Table 5. Group I. 4. 6/8/6-Taxanes. Taxa-4(20),11-dienes oxygenated at C-14.
_________________________________________________________________________________________________________________
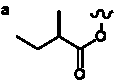
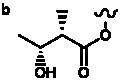
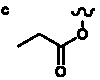
| Abbreviations: |
| OAc, O-acetyl |
| OEt, O-ethyl |
| OGlc, O-β-D-glucopyranosyl |
| OMe, O-methyl |
_________________________________________________________________________________________________________________
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
R7 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-2 |
C-5 |
C-7 |
C-9 |
C-10 |
C-13 |
C-14 |
Record |
Source(s) |
|
| ____________________________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxuyunnanine J |
C22H34O4 |
362.2457 |
A |
H |
OAc |
H |
H |
OH |
H |
OH |
3578 |
T. yunnanensis |
Zhang et al. (1995d) |
| 10β,13α-Diacetoxytaxa-4(20),11-diene- |
C24H36O7 |
436.2461 |
A |
H |
OH |
H |
OH |
OAc |
OAc |
OH |
137 |
T. canadensis |
Shi et al. (2007) |
| 5α,9α,14β-triol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 14β-Hydroxytaxusin |
C28H40O9 |
520.2672 |
A |
H |
OAc |
H |
OAc |
OAc |
OAc |
OH |
907 |
T. mairei |
Shi et al. (1998a) |
| 5α,9α,10β,13α-Tetraacetoxy-14β-(β-D- |
C34H50O14 |
682.3201 |
A |
H |
OAc |
H |
OAc |
OAc |
OAc |
OGlc |
136 |
T. baccata |
Shi et al. (2004a) |
| glucopyranosyloxy)taxa-4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 9α,10β-Diacetoxytaxa-4(20),11-diene- |
C24H36O7 |
436.2461 |
A |
OH |
OH |
H |
OAc |
OAc |
H |
OH |
135 |
T. mairei |
Shen et al (2004) |
| 2α,5α,14β-triol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,10β,14β-Trihydroxy-5α-acetoxytaxa- |
C22H34O5 |
378.2406 |
A |
OH |
OAc |
H |
H |
OH |
H |
OH |
3674 |
T. mairei |
Cheng et al. (1996) |
| 4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 5α,10β,14β-Triacetoxytaxa-4(20),11-dien- |
C26H38O7 |
462.2618 |
A |
OH |
OAc |
H |
H |
OAc |
H |
OAc |
3675 |
T. mairei |
Cheng et al. (1996) |
| 2α-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,14β-Diacetoxy-10β-methoxytaxa- |
C25H38O6 |
434.2668 |
A |
OAc |
OH |
H |
H |
OMe |
H |
OAc |
132 |
T. cuspidata |
Dong et al. (2007) |
| 11,4(20)-dien-5α-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α-Acetoxy-10β-ethoxytaxa-11,4(20)-diene- |
C24H38O5 |
406.2719 |
A |
OAc |
OH |
H |
H |
OEt |
H |
OH |
133 |
T. cuspidata |
Dong et al. (2007) |
| 5α,14β-diol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α-Acetoxytaxa-4(20),11-diene-5α,10β,14β-triol |
C22H34O5 |
378.24063 |
A |
OAc |
OH |
H |
H |
OH |
H |
OH |
3398 |
T. cuspidata |
Zhang et al. (2012b) |
| Sinenxan H |
C24H36O6 |
420.2512 |
A |
OAc |
OH |
H |
H |
OH |
H |
OAc |
3837 |
T. cuspidata |
Li et al. (2011) |
| Hongdoushan C |
C27H42O6 |
462.2981 |
A |
OAc |
OH |
H |
H |
OH |
H |
a |
127 |
T. mairei |
Banskota et al. (2002) |
| Tasumatrol K |
C29H44O8 |
520.3036 |
A |
OAc |
OH |
H |
H |
OAc |
H |
b |
128 |
T. sumatrata |
Shen et al (2005a) |
| 2α,5α-Diacetoxy-10β-(6′-O-acetyl-β-d-gluco- |
C37H56O14 |
724.3670 |
A |
OAc |
OAc |
H |
H |
e |
H |
b |
3838 |
T. canadensis |
Li et al. (2015) |
| pyranosyl)oxy-14β-[(2′R,3′S)-3′-hydroxy- |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2′-methylbutanoyl]oxytaxa-4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxuyunnanine H |
C25H38O6 |
434.2668 |
A |
OAc |
OH |
H |
H |
OH |
H |
c |
129 |
T. yunnanensis |
Zhang et al. (1995d) |
| Taxuyunnanine I |
C26H40O6 |
448.2825 |
A |
OAc |
OH |
H |
H |
OH |
H |
d |
130 |
T. yunnanensis |
Zhang et al. (1995d) |
| 2α,14β-Diacetoxy-10β-ethoxy-taxa-11,4(20)- |
C26H40O6 |
448.2825 |
A |
OAc |
OH |
H |
H |
OEt |
H |
OAc |
131 |
T. cuspidata |
Dong et al. (2007) |
| dien-5α-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,14β-Diacetoxy-10β-(β-d-glucopyranosyl)- |
C30H46O11 |
582.3040 |
A |
OAc |
OH |
H |
H |
OGlc |
H |
OAc |
3864 |
T. canadensis |
Li et al. (2015) |
| taxa-11,4(20)-dien-5α-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taiwanxan |
C31H46O9 |
562.3142 |
A |
OAc |
OH |
H |
OAc |
OAc |
H |
a |
3806 |
T. mairei |
Ho et al. (1987) |
| Hongdoushan A |
C29H44O7 |
504.3087 |
A |
OAc |
OAc |
H |
H |
OH |
H |
a |
121 |
T. mairei |
Banskota et al. (2002) |
| Hongdoushan B |
C27H40O7 |
476.2774 |
A |
OAc |
OAc |
H |
H |
OH |
H |
c |
122 |
T. mairei |
Banskota et al. (2002) |
| Taxuyunnanine G |
C24H36O6 |
420.2512 |
A |
OAc |
OAc |
H |
H |
OH |
H |
OH |
123 |
T. yunnanensis |
Zhang et al. (1995d) |
| 10-Deacetylyunnanaxane |
C29H44O8 |
520.3036 |
A |
OAc |
OAc |
H |
H |
OH |
H |
b |
124 |
T. media |
Gabetta et al. (1995a) |
| 2α,5α-Diacetoxy-10β-methoxy-14β-(2- |
C30H46O7 |
518.3244 |
A |
OAc |
OAc |
H |
H |
OMe |
H |
a |
906 |
T. baccata |
Topcu et al. (1994) |
| methylbutanoyloxy)taxa-4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxuyunnanine C |
C28H40O8 |
504.2723 |
A |
OAc |
OAc |
H |
H |
OAc |
H |
OAc |
930 |
T. yunnanensis |
Zhang et al. (1994) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. mairei |
Ma et al. (1994a) |
| 2α,5α,10β-Triacetoxy-14β-(2-methyl- |
C31H46O8 |
546.3193 |
A |
OAc |
OAc |
H |
H |
OAc |
H |
a |
28 |
T. yunnanensis |
Banskota et al. (2002) |
| butanoyloxy)taxa-4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
T. baccata |
Erdemoglu et al. (2001) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. cuspidata |
Sugiyama et al. (1994) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. mairei |
Ma et al. (1994a) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. wallichiana |
Chattopadhyay et al. (1996) |
| Yunnanxane |
C31H46O9 |
562.3142 |
A |
OAc |
OAc |
H |
H |
OAc |
H |
b |
43 |
T. yunnanensis |
Chen et al. (1991) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. mairei |
Ma et al. (1994a) |
| 2α,5α,10β-Triacetoxy-14β-propionyloxy- |
C29H42O8 |
518.2880 |
A |
OAc |
OAc |
H |
H |
OAc |
H |
c |
3711 |
T. mairei |
Ma et al. (1994a) |
| taxa-4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,5α,10β-Triacetoxytaxa-14β-isobutyryl- |
C30H44O8 |
532.30362 |
A |
OAc |
OAc |
H |
H |
OAc |
H |
d |
3721 |
T. chinensis |
Ma et al. (1994a) |
| oxytaxa-4(20),11-dien-7β-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,5α,14β-Triacetoxy-10β-(β-D-gluco- |
C32H48O12 |
624.3146 |
A |
OAc |
OAc |
H |
H |
OGlc |
H |
OAc |
119 |
T. canadensis |
Shi et al. (2003a) |
| pyranosyl)taxa-4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,5α-Diacetoxy-10β-(β-D-gluco- |
C35H54O12 |
666.3615 |
A |
OAc |
OAc |
H |
H |
OGlc |
H |
a |
118 |
T. canadensis |
Shi et al. (2003a) |
| pyranosyl)-14β-[(S)-2'-methyl- |
|
|
|
|
|
|
|
|
|
|
|
|
|
| butanoyloxy]taxa-4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-Deacetyl-10β-(β-D-glucopyranosyl) |
C29H44O7 |
504.3087 |
A |
OAc |
OAc |
H |
H |
OGlc |
H |
b |
120 |
T. yunnanensis |
Li et al. (2003) |
| yunnanxane |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,5α,10β-Triacetoxy-14β-(2-methyl- |
C31H46O9 |
562.3142 |
A |
OAc |
OAc |
H |
OH |
OAc |
H |
a |
125 |
T. mairei |
Yang et al. (1996) |
| butanoyloxy)taxa-4(20),11-dien-9α-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,9α,10β-Triacetoxytaxa-4(20),11-diene- |
C26H38O8 |
478.2567 |
A |
OAc |
OH |
H |
OAc |
OAc |
H |
OH |
134 |
T. mairei |
Shen et al (2004) |
| 5α,14β-diol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,5α,9α,10β,14β-Pentaacetoxytaxa- |
C30H42O10 |
562.2778 |
A |
OAc |
OAc |
H |
OAc |
OAc |
H |
OAc |
126 |
T. mairei |
Yang et al. (1996) |
| 4(20),11-diene |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxuyunnanine B |
C33H48O10 |
604.3247 |
A |
OAc |
OAc |
H |
OAc |
OAc |
H |
a |
931 |
T. yunnanensis |
Zhang et al. (1994a) |
| 2α,5α,10β,14β-Tetraacetoxytaxa- |
C28H40O9 |
520.2672 |
A |
OAc |
OAc |
OH |
H |
OAc |
H |
OAc |
3722 |
T. cuspidata |
Fedoreyev et al. (1998) |
| 4(20),11-dien-7β-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| ___________________________________________________________________________________________________________________________________________________________________________________ |
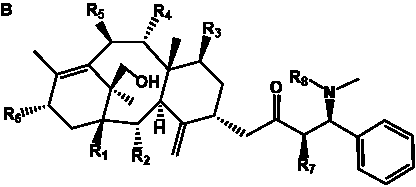
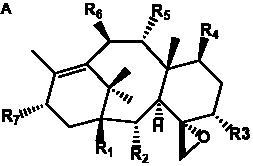
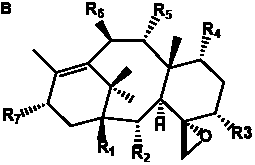
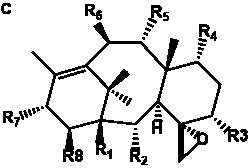
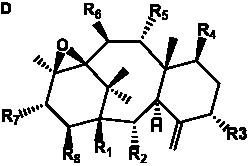
Table 6. Group I.5. 6/8/6-Taxanes. Tax-4-enes with a C-11,2-epoxide and tax-11-enes with C-4,20-epoxide functional group.
_________________________________________________________________________________________________________________
| Abbreviations: |
| OAc, O-acetyl |
| OEt, O-ethyl |
| OGlc, O-β-D-glucopyranosyl |
|
| Symbols: |
| =O, ketone functional group |
| ________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
R7 |
R8 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-1 |
C-2 |
C-5 |
C-7 |
C-9 |
C-10 |
C-13 |
C-14 |
Record |
Source(s) |
|
| ________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| C-4,20-epoxides with hydroxyl and acetyl functional groups |
| 10β,13α-Diacetoxy-4β,20-epoxytax-11-ene- |
C24H36O8 |
452.2410 |
A |
H |
OH |
OH |
H |
OH |
OAc |
OAc |
H |
180 |
T. chinensis |
Zhang et al. (1995a); |
| 2α,5α,9α-triol |
|
|
|
|
|
|
|
|
|
|
|
|
|
Wiedenfeld et al. (1995) |
| 1β-Hydroxy-2,7,9-trideacetylbaccatin I |
C26H38O11 |
526.2414 |
A |
OH |
OH |
OAc |
OH |
OH |
OAc |
OAc |
H |
912 |
T. yunnanensis |
Chen et al. (1994a) |
| Taxumariene F |
C28H40O12 |
568.25198 |
A |
OH |
OAc |
OH |
OH |
OAc |
OAc |
OAc |
H |
1075 |
T. mairei |
Chen et al. (2020) |
| Taxumariene G |
C28H40O12 |
568.25198 |
A |
OH |
OAc |
OH |
OAc |
OH |
OAc |
OAc |
H |
1076 |
T. mairei |
Chen et al. (2020) |
| Taxumairol C |
C26H38O11 |
526.2414 |
A |
OH |
OAc |
OAc |
OH |
OH |
OH |
OAc |
H |
181 |
T. mairei |
Shen and Chen (1997) |
| 2α,5α,10β-Triacetoxy-4β,20-epoxytax-11-ene- |
C26H28O11 |
516.16316 |
A |
OH |
OAc |
OAc |
OH |
OH |
OAc |
OH |
H |
1074 |
T. canadensis |
Yao et al. (2013) |
| 1β,7β,9α,13α-tetraol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxumairol B |
C28H40O12 |
568.2520 |
A |
OH |
OAc |
OAc |
OH |
OH |
OAc |
OAc |
H |
182 |
T. mairei |
Shen et al. (1996) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
T. canandensis |
Zamir at al. (1995) |
| 1β-Hydroxy-2α,7β-deacetylbaccatin I |
C28H40O12 |
568.2520 |
A |
OH |
OH |
OAc |
OH |
OAc |
OAc |
OAc |
H |
965 |
T. chinensis |
Wang et al. (2003) |
| 5α-Deacetylbaccatin I |
C30H42O12 |
568.2520 |
A |
H |
OAc |
OH |
OAc |
OAc |
OAc |
OAc |
H |
967 |
T. baccata |
Della Casa de Marcano and |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Halsall (1970) |
| Taxuspine V |
C30H42O13 |
610.2625 |
A |
OH |
OAc |
OH |
OAc |
OAc |
OAc |
OAc |
H |
185 |
T. cuspidata |
Hosoyama et al. (1996) |
| 2α,5α,9α,10β,13α-Pentaacetoxy-4β,20- |
C30H42O13 |
610.2625 |
A |
OH |
OAc |
OAc |
OH |
OAc |
OAc |
OAc |
H |
183 |
T. brevifolia |
Chu et al. (1993b) |
| epoxytax-11-ene-1β,7β-diol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,5α,7β,10β,13α-Pentaacetoxy-4β,20- |
C30H42O13 |
610.2625 |
A |
OH |
OAc |
OAc |
OAc |
OH |
OAc |
OAc |
H |
184 |
T. brevifolia |
Chu et al. (1993b) |
| epoxytax-11-ene-1β,9α-diol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxumairol D |
C32H44O13 |
636.2782 |
A |
OH |
OAc |
OAc |
OAc |
OAc |
OH |
OAc |
H |
186 |
T. yunnanensis |
Zhang et al. (1997) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
T. mairei |
Shen and Chen (1997) |
| Taxumairol F |
C30H42O13 |
636.2782 |
A |
OH |
OAc |
OAc |
OAc |
OAc |
OAc |
OH |
H |
958 |
T. mairei |
Chauvière et al. (1981) |
| Baccatin I |
C32H44O14 |
652.2731 |
A |
H |
OAc |
OAc |
OAc |
OAc |
OAc |
OAc |
H |
187 |
T. baccata |
Della Casa de Marcano and |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Halsall (1970) |
| 1β-Hydroxybaccatin I |
C32H44O14 |
652.2731 |
A |
OH |
OAc |
OAc |
OAc |
OAc |
OAc |
OAc |
H |
188 |
T. baccata |
Della Casa de Marcano and |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Halsall (1970) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
T. wallichiana |
Viterbo et al. (1997) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
T. mairei |
Yeh et a. (1988) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
T. chinensis |
Wang et al. (2003a) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
T. cuspidata |
Mao et al. (1999) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
T. yunnanensis |
Xiao et al. (1994) |
| 1β-Acetoxy-5α-deacetylbaccatin I |
C32H44O14 |
652.2731 |
A |
OAc |
OAc |
OH |
OAc |
OAc |
OAc |
OAc |
H |
189 |
T. mairei |
Lian et al. (1988) |
| 1β-Acetoxybaccatin I |
C34H46O15 |
694.2837 |
A |
OAc |
OAc |
OAc |
OAc |
OAc |
OAc |
OAc |
H |
966 |
T. yunnanensis |
Liang et al. (2002) |
| 1β,7α-Dihydroxy-7β-deacetoxybaccatin I |
C30H42O13 |
610.2625 |
B |
OH |
OAc |
OAc |
OH |
OAc |
OAc |
OAc |
H |
176 |
T. baccata |
Sénilh et al. (1984) |
| 9α,10β-Diacetoxy-4β,20-epoxytax-11-ene- |
C24H36O8 |
452.2410 |
C |
H |
OH |
OH |
OAc |
OAc |
OAc |
OAc |
OH |
177 |
T. mairei |
Shen et al. (2004) |
| 2α,5α,14β-triol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| C-4,20-epoxides with assorted functional groups |
| 2α,7β,9α,10β,13α-Pentaacetoxy-5α-cinnamoyl- |
C39H48O14 |
740.3044 |
A |
OH |
OAc |
a |
OAc |
OAc |
OAc |
OAc |
H |
179 |
T. cuspidata |
Shi et al. (1999e) |
| oxy-4β,20-epoxytax-11-en-1β-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 1β-Hydroxy-10β-deacetyl-10β-glycolyl- |
C32H44O15 |
668.2680 |
A |
OH |
OAc |
OAc |
OAc |
OAc |
b |
OAc |
H |
178 |
T. canadensis |
Zhang et al. (2000b) |
| baccatin I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 7β-Acetoxy-9-acetylspicataxine |
C41H55N1O13 |
769.3673 |
A |
H |
OAc |
c |
OAc |
OAc |
OAc |
OAc |
H |
190 |
T. media |
Appendino et al. (1994b) |
| C-11,12-epoxides |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxinine A-11,12-epoxide |
C26H36O9 |
492.2359 |
D |
H |
OAc |
OH |
H |
OAc |
OAc |
=O |
H |
154 |
T. cuspidata |
Murakami et al. (1999a) |
| Decinnamoyltaxinine B-11,12-oxide |
C28H38O11 |
550.2414 |
D |
H |
OAc |
OH |
OAc |
OAc |
OAc |
=O |
H |
156 |
T. yunnanensis |
Yue et al. (1996) |
| 2α,9α,10β-Triacetoxy-11,12-epoxy-5α- |
C32H46O14 |
654.288756 |
D |
H |
OAc |
OGlc |
H |
OAc |
OAc |
=O |
H |
959 |
T. cuspidata |
Wang et al. (2005) |
| (β-D-glucopyranosyloxy)tax-4(20)-en-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,10β-Diacetoxy-5α-cinnamoyloxy-11,12- |
C33H40O9 |
580.2672 |
D |
H |
OAc |
a |
H |
OH |
OAc |
=O |
H |
961 |
T. cuspidata |
Shi et al. (2006b) |
| epoxy-9α-hydroxytax-4(20)-en-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,9α-Diacetoxy-5α-cinnamoyloxy-11,12-epoxy- |
C33H40O9 |
580.2672 |
D |
H |
OAc |
a |
H |
OAc |
OH |
=O |
H |
960 |
T. cuspidata |
Shi et al. (2006b) |
| 10β-hydroxytax-4(20)-en-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,7β,9α-Triacetoxy-5α-cinnamoyloxy-11,12- |
C35H42O11 |
638.2727 |
D |
H |
OAc |
a |
OH |
OAc |
OH |
=O |
H |
1086 |
T. canadensis |
Shi et al. (2003d) |
| epoxy-10β-hydroxytax-4(20)-en-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Dantaxusin C |
C37H44O12 |
680.2833 |
D |
H |
OAc |
a |
OAc |
OAc |
OAc |
=O |
H |
155 |
T. yunnanensis |
Shinozaki et al. (2002) |
| 7β,9α-Diacetoxy-2α-benzoyloxy-11,12-epoxy |
C33H40O13 |
644.24689 |
D |
OH |
OBz |
OH |
OAc |
OAc |
b |
=O |
H |
1087 |
T. cuspidata |
Zhang et al. (2012b) |
| -1β,5α-dihydroxy-10β-glycolyl- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| tax-4(20)-en-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ________________________________________________________________________________________________________________________________________________________________ |
Table 7. Group I.6. 6/8/6-Taxanes with an oxetane ring.
_________________________________________________________________________________________________________________
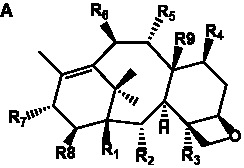
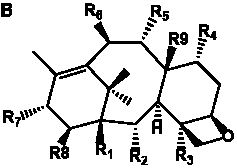
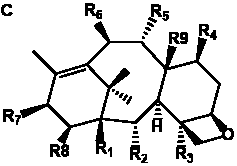
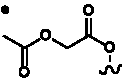
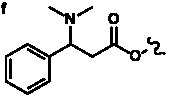
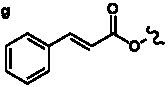
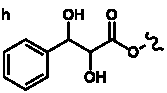
| Abbreviations: |
| OAc, O-acetyl |
| OEt, O-ethyl |
| OGlc, O-β-D-glucopyranosyl |
| OXyl, O-β-D-xylopyranosyl |
|
| Symbols: |
| =O, ketone functional group |
| ____________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
R7 |
R8 |
R9 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-1 |
C-2 |
C-4 |
C-7 |
C-9 |
C-10 |
C-13 |
C-14 |
C-19 |
Record |
Source(s) |
|
| ____________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Normal 6/8/6 taxanes with an oxetane ring and hydroxyl, acetyl and/or benzoyl functional groups on C-1, C-2, C-5, C-7, C-9, C-10 and/or C-13 |
| 1β-Dehydroxy-4α-deacetyl- |
C30H42O12 |
594.2676 |
A |
H |
OAc |
OH |
OAc |
OAc |
OAc |
OAc |
H |
H |
214 |
T. mairei |
Lian et al. (1988) |
| baccatin IV |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 1-Dehydroxybaccatin IV |
C32H44O13 |
636.2782 |
A |
H |
OAc |
OAc |
OAc |
OAc |
OAc |
OAc |
H |
H |
202 |
not mentioned |
Della Casa de Marcano and Halsall |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1975) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
T. mairei |
Min et al. (1989) |
| 1-Dehydroxybaccatin III |
C31H37O10 |
569.2391 |
A |
H |
OBz |
OAc |
OH |
=O |
OAc |
OH |
H |
H |
193 |
T. yunnanensis |
Zhang and Jia (1990) |
| 1-Dehydroxybaccatin VI |
C37H46O13 |
698.2938 |
A |
H |
OBz |
OAc |
OAc |
OAc |
OAc |
OAc |
H |
H |
42 |
T. mairei |
Min et al. (1989) |
| 2-Deacetylbaccatin IV |
C30H42O13 |
610.2625 |
A |
OH |
OH |
OAc |
OAc |
OAc |
OAc |
OAc |
H |
H |
252 |
T. media |
Fischedick et al. (2015) |
| 7,9-Deacetylbaccatin IV |
C28H40O12 |
568.2520 |
A |
OH |
OAc |
OAc |
OH |
OH |
OAc |
OAc |
H |
H |
201 |
T. canadensis |
Zamir et al. (1992) |
| Baccatin IV |
C32H44O14 |
652.2731 |
A |
OH |
OAc |
OAc |
OAc |
OAc |
OAc |
OAc |
H |
H |
203 |
not mentioned |
Della Casa de Marcano and Halsall |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1975) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
T. chinensis |
Wang et al. (2003b) |
| 4-Deacetylbaccatin IV |
C30H42O13 |
610.2625 |
A |
OH |
OBz |
OH |
OAc |
OAc |
OAc |
OAc |
H |
H |
215 |
T. media |
Appendino et al. (1994b) |
| 7,9,10-Deacetylbaccatin VI |
C31H41O11 |
588.2571 |
A |
OH |
OBz |
OAc |
OH |
OH |
OH |
OAc |
H |
H |
217 |
T. canadensis |
Zamir et al. (1995a) |
| Taxuspinanane C |
C29H36O10 |
544.2308 |
A |
OH |
OBz |
OAc |
OH |
OH |
OH |
=O |
H |
H |
216 |
T. cuspidata |
Morita et al. (1997a) |
| 9-Dihydrobaccatin III |
C31H40O11 |
588.2571 |
A |
OH |
OBz |
OAc |
OH |
OH |
OAc |
OH |
H |
H |
27 |
T. canadensis |
Ketchum et al. (1999) |
| 9-Dihydro-13-acetylbaccatin III |
C33H42O12 |
630.2676 |
A |
OH |
OBz |
OAc |
OH |
OH |
OAc |
OAc |
H |
H |
44 |
T. canadensis |
Gunawardana et al. (1992) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
T. mairei |
Zhou et al. (2002) |
| Taxuspinanane D |
C31H38O11 |
586.2414 |
A |
OH |
OBz |
OAc |
OH |
OH |
OAc |
=O |
H |
H |
222 |
T. cuspidata |
Morita et al. (1998b) |
| 10-Deacetyl-13-oxobaccatin III |
C29H33O10 |
541.2074 |
A |
OH |
OBz |
OAc |
OH |
=O |
OH |
=O |
H |
H |
903 |
T. sumatrana |
Kitagawa et al. (1995) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
T. cuspidata |
Zhang et al. (2010) |
| 10-Deacetylbaccatin III |
C29H35O10 |
543.2236 |
A |
OH |
OBz |
OAc |
OH |
=O |
OH |
OH |
H |
H |
29 |
T. baccata |
Chauvière et al. (1981) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
T. yunnanensis |
Zhang and Jia (1990) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
T. brevifolia |
Kingston et al. (1982) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
T. canadensis |
Zamir et al. (1992) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
T. chinensis |
Fuji et al. (1993) |
| Baccatin III |
C31H38O11 |
586.2414 |
A |
OH |
OBz |
OAc |
OH |
=O |
OAc |
OH |
H |
H |
30 |
T. baccata |
Della Casa de Marcano et al. (1970a) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
reported wrong structure; corrected by |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Della Casa de Marcano and Halsall |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1975) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
T. wallichiana |
Miller et al. (1981) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
T. mairei |
Shen and Chen (1997) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
T. chinensis |
Fuji et al. (1993) |
| 13-Oxobaccatin III |
C31H46O11 |
594.3040 |
A |
OH |
OBz |
OAc |
OH |
=O |
OAc |
=O |
H |
H |
218 |
T. cuspidata |
Zhang et al. (2010) |
| Taxuspine E |
C31H40O11 |
588.2571 |
A |
OH |
OBz |
OAc |
OAc |
OH |
OH |
OH |
H |
H |
195 |
T. cuspidata |
Kobayashi et al. (1995) |
| 9-Deacetylbaccatin VI |
C35H44O13 |
672.2782 |
A |
OH |
OBz |
OAc |
OAc |
OH |
OAc |
OAc |
H |
H |
204 |
T. yunnanensis |
Zhang et al. (1997) |
| 10-Deacetylbaccatin VI |
C35H44O13 |
672.2782 |
A |
OH |
OBz |
OAc |
OAc |
OAc |
OH |
OAc |
H |
H |
205 |
T. yunnanensis |
Zhang et al. (1997) |
| Baccatin VI |
C37H46O14 |
714.2888 |
A |
OH |
OBz |
OAc |
OAc |
OAc |
OAc |
OAc |
H |
H |
1039 |
not mentioned |
Della Casa de Marcano and Halsall |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1975) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
T. mairei |
Min et al. (1989) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
T. baccata |
Sénilh et al. (1984) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
T. chinensis |
Fuji et al. (1993) |
| 1β-Acetylbaccatin IV |
C34H46O15 |
694.2837 |
A |
OAc |
OAc |
OAc |
OAc |
OAc |
OAc |
OAc |
H |
H |
200 |
T. yunnanensis |
Zhang and Jia (1990) |
| 1β-Acetyl-10-deacetylbaccatin III |
C31H38O11 |
586.2414 |
A |
OAc |
OBz |
OAc |
OH |
=O |
OH |
OH |
H |
H |
1035 |
T. canadensis |
Zamir et al. (1992) |
| 7-epi-9,10-Deacetylbaccatin VI |
C31H41O11 |
588.2571 |
B |
OH |
OBz |
OAc |
OH |
OH |
OH |
OAc |
H |
H |
1038 |
T. canadensis |
Zamir et al. (1995a) |
| Baccatin V |
C31H38O11 |
586.2414 |
B |
OH |
OBz |
OAc |
OH |
=O |
OAc |
OH |
H |
H |
194 |
T. baccata |
Della Casa de Marcano et al. (1970a) |
| 10-Deacetyl-10-oxobaccatin V |
C29H34O10 |
542.2152 |
B |
OH |
OBz |
OAc |
OH |
=O |
=O |
OH |
H |
H |
192 |
T. chinensis |
Fuji et al. (1993) |
| 13-epi-10-Deacetylbaccatin III |
C29H35O10 |
543.2236 |
C |
OH |
OBz |
OAc |
OH |
=O |
OH |
OH |
H |
H |
223 |
T. baccata |
Gabetta et al. (1995b) |
| Taxadienes with an oxetane ring and substituents other than hydroxyl, acetyl or benzoyl functional groups |
| 10-Deacetyl-10β-O-glycolyl- |
C32H44O15 |
668.2680 |
A |
OH |
OAc |
OAc |
OAc |
OAc |
c |
OAc |
H |
H |
199 |
T. canadensis |
Zhang et al. (2000) |
| baccatin IV |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxuspine N |
C46H57N1O14 |
847.3779 |
A |
OH |
OAc |
OAc |
OAc |
OAc |
OBz |
f |
H |
H |
225 |
T. cuspidata |
Kobayashi et al. (1996) |
| Taxuspinanane J |
C40H44O12 |
716.2833 |
A |
OH |
OBz |
OAc |
OH |
=O |
OAc |
g |
H |
H |
224 |
T. mairei |
Morita et al. (1998a) |
| Yunnanxol |
C40H46O14 |
750.2888 |
A |
OH |
OBz |
OAc |
OH |
=O |
OAc |
h |
H |
H |
971 |
T. yunnanensis |
Chen et al. (1993a, 1994b) |
| 10β-(β-Hydroxybutyrl)- |
C33H42O12 |
630.2676 |
A |
OH |
OBz |
OAc |
OH |
=O |
d |
OH |
H |
H |
196 |
T. baccata |
Soto et al. (1998) |
| 10-deacetylbaccatin III |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (10β-O-Acetylglycolyl)baccatin IV |
C39H48O16 |
772.2942 |
A |
OH |
OBz |
OAc |
OAc |
OAc |
e |
OAc |
H |
H |
198 |
T. canadensis |
Zhang et al. (2001) |
| 10-Hydroxyacetylbaccatin VI |
C37H46O15 |
730.2837 |
A |
OH |
OBz |
OAc |
OAc |
OAc |
c |
OAc |
H |
H |
206 |
T. canadensis |
Zamir et al. (1995a) |
| 7β-(β-D-Xylopyranosyl)- |
C34H44O14 |
676.2731 |
A |
OH |
OBz |
OAc |
OXyl |
=O |
OH |
OH |
H |
H |
913 |
T. yunnanensis |
Chen et al. (1994a) |
| 10-deacetylbaccatin III |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 7β-(β-D-Xylopyranosyl)baccatin III |
C36H46O15 |
718.2837 |
A |
OH |
OBz |
OAc |
OXyl |
=O |
OAc |
OH |
H |
H |
968 |
T. yunnanensis |
Li et al. (1998) |
| 2-Tigloyl-2-debenzoyl- |
C27H38O10 |
522.2465 |
A |
OH |
a |
OAc |
OH |
=O |
OH |
OH |
H |
H |
191 |
T. baccata |
Gabetta et al. (1995b) |
| 10-deacetylbaccatin III |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Baccatin VII |
C36H52O14 |
708.3357 |
A |
OH |
b |
OAc |
OAc |
OAc |
OAc |
OAc |
H |
H |
969 |
not mentioned |
Della Casa de Marcano and Halsall |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1975) |
| Taxadienes with an oxetane ring and a C-14 substituent |
| 14β-Hydroxy-10-deacetyl- |
C22H32O10 |
456.19955 |
A |
H |
OH |
H |
OH |
=O |
OH |
OH |
OH |
H |
4149 |
T. chinensis |
Xia et al. (2005a) |
| 2-O-debenzoylbaccatin III |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,14β-Dihydroxy-2-deacetyl- |
C28H40O11 |
552.2571 |
A |
H |
OH |
H |
OAc |
OAc |
OAc |
OAc |
OH |
H |
3938 |
T. chinensis |
Wang et al. (2004b) |
| baccatin IV |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 14β-Benzoyloxy-2-deacetyl- |
C37H46O15 |
730.2837 |
A |
H |
OH |
H |
OAc |
OAc |
OAc |
OAc |
OBz |
H |
212 |
T. chinensis |
Wang et al. (2004a) |
| baccatin IV |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 14β-Benzoyloxy-13-deacetyl- |
C37H46O15 |
730.2837 |
A |
H |
OAc |
H |
OAc |
OAc |
OAc |
OH |
OBz |
H |
211 |
T. chinensis |
Wang et al. (2004a) |
| baccatin IV |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 14β-Benzoyloxybaccatin IV |
C39H48O16 |
772.2942 |
A |
H |
OAc |
H |
OAc |
OAc |
OAc |
OAc |
OBz |
H |
210 |
T. chinensis |
Wang et al. (2004a) |
| 14β-Hydroxybaccatin VI |
C37H46O15 |
730.2837 |
A |
H |
OBz |
H |
OAc |
OAc |
OAc |
OAc |
OH |
H |
213 |
T. chinensis |
Wang et al. (2003b) |
| 14β-Hydroxy-10-deacetyl- |
C29H36O11 |
560.2258 |
A |
H |
OBz |
OAc |
OH |
=O |
OH |
OH |
OH |
H |
970 |
T. wallichiana |
Appendino et al. (1992b) |
| baccatin III |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Baccatin X |
C31H40O12 |
604.25198 |
A |
OH |
OH |
OAc |
OH |
OH |
OAc |
OH |
OBz |
H |
3397 |
T. yunnanensis |
Hai et al. (2014) |
| 14β-Benzoyloxy-2-debenzoyl- |
C29H36O11 |
560.2258 |
A |
OH |
OH |
OAc |
OH |
=O |
OH |
OH |
OBz |
H |
886 |
T. wallichiana |
Appendino et al. (1993e) |
| 10-deacetylbaccatin III |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Baccatin IX |
C31H40O12 |
604.25198 |
A |
OH |
OBz |
OAc |
OH |
OH |
OAc |
OH |
OH |
H |
3396 |
T. yunnanensis |
Hai et al. (2014) |
| Baccatin VIII |
C33H42O13 |
646.26255 |
A |
OH |
OBz |
OAc |
OH |
OH |
OAc |
OAc |
OH |
H |
3395 |
T. yunnanensis |
Hai et al. (2014) |
| Taxadienes with an oxetane ring and a C-19 substituent |
| 9(βH)-9-Dihydro-19-acetoxy- |
C31H40O12 |
604.2520 |
A |
H |
OBz |
OAc |
OH |
OH |
OH |
OH |
H |
OAc |
895 |
T. baccata |
Appendino et al. (1993d) |
| 10-deacetylbaccatin III |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 19-Acetoxy-7,9,10- |
C33H42O13 |
646.26254 |
A |
OH |
OBz |
OAc |
OH |
OH |
OH |
OAc |
H |
OAc |
4363 |
T. baccata |
Ding et al. (2020) |
| deacetylbaccatin VI |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 19-Hydroxybaccatin III |
C31H38O12 |
602.2363 |
A |
OH |
OBz |
OAc |
OH |
=O |
OAc |
OH |
H |
OH |
220 |
T. baccata |
Chauvière et al. (1981) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
T. yunnanensis |
Zhang and Jia (1990) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
T. wallichiana |
McLaughlin et al. (1981) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
T. chinensis |
Fuji et al. (1993) |
| 19-Hydroxy-13-oxobaccatin III |
C31H36O12 |
600.2207 |
A |
OH |
OBz |
OAc |
OH |
=O |
OAc |
=O |
H |
OH |
219 |
T. sumatrana |
Kitagawa et al. (1995) |
| 7-epi-19-Hydroxybaccatin III |
C31H38O12 |
602.2363 |
B |
OH |
OBz |
OAc |
OH |
=O |
OAc |
OH |
H |
OH |
221 |
T. chinensis |
Fuji et al. (1993) |
| Taxadienes with an oxetane ring for which corrected structures have been proposed |
| 13-Deacetylbaccatin VI |
C35H44O13 |
672.2782 |
A |
OH |
OBz |
OAc |
OAc |
OAc |
OAc |
OH |
H |
H |
197 |
T. wallichiana |
Barboni et al. (1993) |
| 4α,7β,9α,13α-Tetraacetoxy- |
C42H48O14 |
776.3044 |
A |
OH |
OBz |
OAc |
OAc |
OAc |
OBz |
OAc |
H |
H |
208 |
T. brevifolia |
Chu et al. (1992) |
| 2α,10β-dibenzoyloxy-5β,20- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
corrected structure proposed |
| epoxytax-11-en-1β-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
by Huang et al. (1998) |
| 4α,7β,13α-Triacetoxy- |
C47H50O14 |
838.3201 |
A |
OH |
OBz |
OAc |
OAc |
OBz |
OBz |
OAc |
H |
H |
209 |
T. brevifolia |
Chu et al. (1992) |
| 2α,9α,10β-tribenzoyloxy- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
corrected structure proposed |
| 5β,20-epoxytax-11-en-1β-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
by Huang et al. (1998) |
| 4α,9α,10β,13α-Tetraacetoxy- |
C42H48O14 |
776.3044 |
A |
OH |
OBz |
OAc |
OBz |
OAc |
OAc |
OAc |
H |
H |
207 |
T. brevifolia |
Chu et al. (1992) |
| 2α,7β-dibenzoyloxy-5β,20- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
corrected structure proposed |
| epoxytax-11-en-1β-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
by Huang et al. (1998) |
| ____________________________________________________________________________________________________________________________________________________________ |
Table 8. Group I.7. 6/8/6-Taxanes with and oxetane ring and C-13 phenylisoserine side chain.
_________________________________________________________________________________________________________________
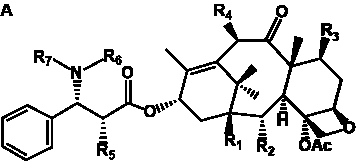
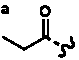
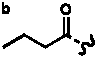
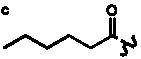
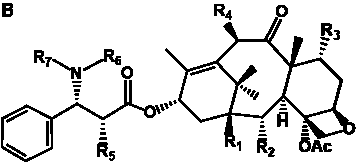
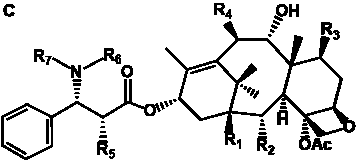
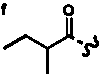
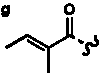
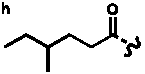
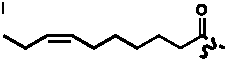
| Abbreviations: |
| Ac, acetyl |
| OAc, O-acetyl |
| Bz, benzyl |
| OBz, O-benzoyl |
| OXyl, O-β-D-xylopyranosyl |
|
| Symbols: |
| =O, ketone functional group |
| ___________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
R7 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-1 |
C-2 |
C-7 |
C-10 |
C-2' |
|
|
Record |
Source(s) |
|
| ___________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 6/8/6-Taxanes with a short- or medium-chain aliphatic amide moiety as part of C-13 side chain |
| N-Debenzoyl-N-propanoyl-10-deacetyltaxol |
C41H49NO13 |
763.3204 |
A |
OH |
OBz |
OH |
OH |
OH |
H |
a |
228 |
T. baccata |
Gabetta et al. (1998) |
| N-Debenzoyl-N-butanoyl-10-deacetyltaxol |
C42H51NO13 |
777.3360 |
A |
OH |
OBz |
OH |
OH |
OH |
H |
b |
229 |
T. baccata |
Gabetta et al. (1998) |
| 10-Deacetyltaxuyunnanine A |
C44H55NO13 |
805.3673 |
A |
OH |
OBz |
OH |
OH |
OH |
H |
c |
973 |
T. yunnanensis |
Zhang et al. (1994c) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. baccata |
Ma et al. (1994b) |
| N-Acetyl-N-debenzoyltaxol |
C42H49NO14 |
791.3153 |
A |
OH |
OBz |
OH |
OAc |
OH |
H |
Ac |
227 |
T. canadensis |
Zhang et al. (2000b) |
| Taxol D (taxcultine) |
C44H53NO14 |
819.3466 |
A |
OH |
OBz |
OH |
OAc |
OH |
H |
b |
231 |
T. media |
Appendino et al. (1994b) |
| Taxol C (taxuyunnanine) |
C46H57NO14 |
847.3779 |
A |
OH |
OBz |
OH |
OAc |
OH |
H |
c |
40 |
T. media |
Barboni et al. (1994a) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. baccata |
Ma et al. (1994b) |
| N-Methyltaxol C |
C47H59NO14 |
861.3936 |
A |
OH |
OBz |
OH |
OAc |
OH |
CH3 |
c |
234 |
T. media |
Barboni et al. (1994a) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. baccata |
Ma et al. (1994b) |
| N-debenzoyl-N-methyl-N-heptanoyl-taxol |
C48H61NO14 |
875.40921 |
A |
OH |
OBz |
OH |
OAc |
OH |
CH3 |
d |
3976 |
T. wallichiana |
Wang et al. (2016) |
| N-debenzoyl-N-methyl-N-octanoyl-taxol |
C49H63NO14 |
889.42486 |
A |
OH |
OBz |
OH |
OAc |
OH |
CH3 |
e |
3977 |
T. wallichiana |
Wang et al. (2016) |
| 7-(β-Xylosyl)-10-deacetyltaxol D |
C47H59NO17 |
909.3783 |
A |
OH |
OBz |
OXyl |
OH |
OH |
H |
b |
242 |
T. yunnanensis |
Guo et al. (1995a) |
| 7β-(Xylosyl)-10-deacetyltaxol C |
C49H63NO17 |
937.4096 |
A |
OH |
OBz |
OXyl |
OH |
OH |
H |
c |
34 |
T. baccata |
Sénilh et al. (1984) |
| 7-(β-Xylosyl)taxol D |
C49H61NO18 |
951.3889 |
A |
OH |
OBz |
OXyl |
OAc |
OH |
H |
b |
240 |
T. baccata |
Li et al. (2001) |
| 7-(β-Xylosyl)taxol C |
C51H65NO18 |
979.4202 |
A |
OH |
OBz |
OXyl |
OAc |
OH |
H |
c |
244 |
T. baccata |
Sénilh et al. (1984) |
| 7-(β-Xylosyl)-N-methyltaxol C |
C52H67NO18 |
993.4358 |
A |
OH |
OBz |
OXyl |
OAc |
OH |
CH3 |
c |
241 |
T. media |
Li et al. (2001) |
| 7-epi-10-Deacetyltaxuyunnanine A |
C44H55NO13 |
805.3673 |
B |
OH |
OBz |
OH |
OH |
OH |
H |
c |
3866 |
T. cuspidata |
Morita et al. (1998b) |
| (taxuspinanane E) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 7-epi-Taxuyunnanine A |
C46H57NO14 |
847.3779 |
B |
OH |
OBz |
OH |
OAc |
OH |
H |
c |
975 |
T. yunnanensis |
Zhang et al. (1994c) |
| 10-Deacetyl-10-oxo-7-epi-taxuyunnanine A |
C45H47NO13 |
809.3047 |
B |
OH |
OBz |
OH |
=O |
OH |
H |
c |
974 |
T. yunnanensis |
Zhang et al. (1994c) |
| 6/8/6-Taxanes with a branched-chain aliphatic amide moiety as part of C-13 side chain |
| 10-Deacetyltaxol B |
C43H51N1O13 |
789.3360 |
A |
OH |
OBz |
OH |
OH |
OH |
H |
f |
230 |
T. wallichiana |
McLaughlin et al. (1981) |
| Taxol B (cephalomannine) |
C45H53NO14 |
831.3466 |
A |
OH |
OBz |
OH |
OAc |
OH |
H |
f |
37 |
T. wallichiana |
Miller et al. (1981) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. baccata |
Chauvière et al. (1981) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
Sénilh et al. (1984) |
| N-Debenzoyl-N-(2-methylbutyryl)taxol |
C45H55N1O14 |
833.3623 |
A |
OH |
OBz |
OH |
OAc |
OH |
H |
g |
232 |
T. media |
Gabetta et al. (1999) |
| Taxuspinanane A |
C47H59N1O14 |
861.3936 |
A |
OH |
OBz |
OH |
OAc |
OH |
H |
h |
235 |
T. cuspidata |
Morita et al. (1997b) |
| N-Debenzoyl-N-methyl-N-(4-methylhexanoyl)- |
C48H61NO14 |
875.4092 |
A |
OH |
OBz |
OH |
OAc |
OH |
CH3 |
h |
3978 |
T. wallichiana |
Wang et al. (2016) |
| taxol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| N-Debenzoyl-N-methyl-N-(cis-7-decenoyl)- |
C51H65NO14 |
915.4405 |
A |
OH |
OBz |
OH |
OAc |
OH |
CH3 |
i |
3979 |
T. wallichiana |
Wang et al. (2016) |
| taxol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-(β-Hydroxybutyryl)-10-deacetyl- |
C47H57N1O15 |
875.3728 |
A |
OH |
OBz |
OH |
i |
OH |
H |
f |
237 |
T. baccata |
Sénilh et al. (1984) |
| cephalomannine |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 7-(β-Xylosyl)-10-deacetyltaxuspinanane A |
C50H65N1O17 |
951.4252 |
A |
OH |
OBz |
OXyl |
OH |
OH |
H |
h |
245 |
T. cuspidata |
Huo et al. (2007b) |
| 7β-(Xylosyl)cephalomannine |
C50H61N1O18 |
963.3889 |
A |
OH |
OBz |
OXyl |
OAc |
OH |
H |
f |
243 |
T. baccata |
Sénilh et al. (1984) |
| 7-epi-Cephalomannine |
C45H53NO14 |
831.3466 |
B |
OH |
OBz |
OH |
OAc |
OH |
H |
f |
|
T. media |
De Bellis et al. (1995) |
| 2'-Acetyl-7-epi-cephalomannine |
C47H55N1O15 |
873.3572 |
B |
OH |
OBz |
OH |
OAc |
OAc |
H |
f |
249 |
T. canadensis |
Zhang et al. (2001) |
| 10-Deacetyl-10-oxo-7-epi-cephalomannine |
C43H49N1O13 |
787.3204 |
B |
OH |
OBz |
OH |
=O |
OH |
H |
f |
246 |
T. canadensis |
Zhang et al. (2001) |
| 6/8/6-Taxanes with an aromatic amide moiety as part of C-13 side chain |
| 1-Deoxytaxol |
C47H51N1O13 |
837.3360 |
A |
H |
OBz |
OH |
OAc |
OH |
H |
Bz |
251 |
T. mairei |
Shi et al. (2006b) |
| 10-Deacetyltaxol |
C45H49NO13 |
811.3204 |
A |
OH |
OBz |
OH |
OH |
OH |
H |
Bz |
35 |
T. wallichiana |
McLaughlin et al. (1981) |
| Taxol (taxol A; paclitaxel®) |
C47H51NO14 |
853.3310 |
A |
OH |
OBz |
OH |
OAc |
OH |
H |
Bz |
39 |
T. brevifolia |
Wani et al. (1971) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. wallichiana |
Miller et al. (1981) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. baccata |
Sénilh et al. (1984) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. yunnanensis |
Liang et al. (2002) |
| N-Debenzoyl-N-cinnamoyltaxol |
C49H53N1O14 |
897.3572 |
A |
OH |
OBz |
OH |
OAc |
OH |
H |
j |
239 |
T. media |
Gabetta et al. (1995) |
| Taxuspinanane I |
C48H53N1O14 |
867.3466 |
A |
OH |
OBz |
OH |
OAc |
OH |
CH3 |
Bz |
236 |
T. cuspidata |
Morita et al. (1998a) |
| 10-(β-Hydroxybutyryl)-10-deacetyltaxol |
C49H55N1O15 |
875.3728 |
A |
OH |
OBz |
OH |
i |
OH |
H |
Bz |
238 |
T. yunnanensis |
Sénilh et al. (1984) |
| 7β-Acetyl-10-deacetyltaxol |
C47H51N1O14 |
853.3310 |
A |
OH |
OBz |
OAc |
OH |
OH |
H |
Bz |
226 |
T. canadensis |
Zhang et al. (2001) |
| 10-Deacetyl-10-dehydro-7-acetyltaxol A |
C47H49N1O14 |
851.3153 |
A |
OH |
OBz |
OAc |
=O |
OH |
H |
Bz |
233 |
T. media |
Gabetta et al. (1995) |
| 7-(β-Xylosyl)-10-deacetyltaxol |
C50H57NO17 |
943.3626 |
A |
OH |
OBz |
OXyl |
OH |
OH |
H |
Bz |
33 |
T. baccata |
Sénilh et al. (1984) |
| 7-(β-Xylosyl)taxol |
C52H59NO18 |
985.3732 |
A |
OH |
OBz |
OXyl |
OAc |
OH |
H |
Bz |
36 |
T. baccata |
Sénilh et al. (1984) |
| 2-Debenzoyl-2-tigloyltaxol |
C45H53N1O14 |
831.3466 |
A |
OH |
f |
OH |
OAc |
OH |
H |
Bz |
250 |
T. x media |
Gabetta et al. (1999) |
| 7-epi-10-Deacetyltaxol |
C45H49NO13 |
811.3204 |
B |
OH |
OBz |
OH |
OH |
OH |
H |
Bz |
38 |
T. chinensis |
McLaughlin et al. (1981) |
| 7-epi-Taxol |
C47H51NO14 |
853.3310 |
B |
OH |
OBz |
OH |
OAc |
OH |
H |
Bz |
41 |
T. brevifolia |
Huang et al. (1986) |
| 2'-Acetoxy-7-epi-taxol |
C49H53N1O15 |
895.3415 |
B |
OH |
OBz |
OH |
OAc |
OAc |
H |
Bz |
248 |
T. canadensis |
Zhang et al. (2000a) |
| 10-Deacetyl-10-oxo-7-epi-taxol |
C45H47N1O13 |
809.3047 |
B |
OH |
OBz |
OH |
=O |
OH |
H |
Bz |
247 |
T. brevifolia |
Huang et al. (1986) |
| 9-Deoxo-9α-hydroxytaxol |
C47H53NO14 |
855.3466 |
C |
OH |
OBz |
OH |
OAc |
OH |
H |
Bz |
972 |
T. yunnanensis |
Chen et al. (1993a, 1994b) |
| ___________________________________________________________________________________________________________________________________________________ |
Table 9. Group I.8. 6/8/6-Taxanes with opened oxetane ring.
_________________________________________________________________________________________________________________
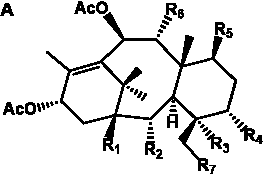
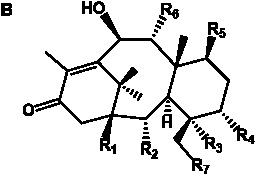
| Abbreviations: |
| OAc, O-acetyl |
| OBz, O-benzoyl |
|
| Symbols: |
| =O, ketone functional group |
| _______________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
R7 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-1 |
C-2 |
C-4 |
C-5 |
C-7 |
C-9 |
C-20 |
Record |
Source(s) |
|
| _______________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 5α,10β,13α-Triacetoxytax-11-ene-2α,7β,9α,20- |
C26H36O10 |
508.2309 |
A |
H |
OH |
H |
OAc |
OH |
OH |
OH |
3923 |
T. cuspidata |
Ni et al. (2011) |
| tetraol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 5α,10β,13α,20-Tetraacetoxytax-11-ene-2α,7β,9α-triol |
C28H42O11 |
554.2727 |
A |
H |
OH |
H |
OAc |
OH |
OH |
OAc |
174 |
T. cuspidata |
Cao et al. (2006) |
| Taxumairol E |
C30H44O12 |
596.2833 |
A |
H |
OH |
H |
OAc |
OAc |
OAc |
OH |
170 |
T. cuspidata |
Shen and Chen (1997a) |
| Taxchin A |
C32H46O13 |
638.2938 |
A |
H |
OH |
H |
OAc |
OAc |
OAc |
OAc |
760 |
T. chinensis |
Li et al. (1993) |
| Taxumairol N |
C28H42O12 |
570.2676 |
A |
H |
OH |
OH |
OH |
OAc |
OAc |
OH |
167 |
T. mairei |
Shen et al. (2000a) |
| Taxumairol A |
C37H48O14 |
716.3044 |
A |
H |
OH |
OH |
OAc |
OAc |
OAc |
OBz |
175 |
T. mairei |
Shen et al. (1996) |
| 7,9-Deacetyltaxuspine L |
C28H42O11 |
554.2727 |
A |
H |
OAc |
H |
OAc |
OH |
OH |
OH |
166 |
T. canadensis |
Zamir et al. (1999a) |
| 7-Deacetyltaxuspine L |
C30H44O12 |
596.2833 |
A |
H |
OAc |
H |
OAc |
OH |
OAc |
OH |
165 |
T. canadensis |
Zhang et al. (2000b) |
| Taxuspine L |
C32H46O13 |
638.2938 |
A |
H |
OAc |
H |
OAc |
OAc |
OAc |
OH |
171 |
T. cuspidata |
Wang et al. (1996b) |
| Taxchin B |
C41H52O14 |
768.3357 |
A |
H |
OAc |
H |
OAc |
OAc |
OAc |
a |
761 |
T. chinensis |
Fuji et al. (1995) |
| Taxumairol L |
C32H46O14 |
654.2888 |
A |
H |
OAc |
OH |
OH |
OAc |
OAc |
OAc |
169 |
T. mairei |
Shen et al. (2002a) |
| 7β,9α,10β,13α,20-Pentaacetoxy-2α-benzoyloxytax- |
C37H48O14 |
716.3044 |
A |
H |
OBz |
OH |
OH |
OAc |
OAc |
OAc |
173 |
T. mairei |
Liang and Kingston (1993) |
| 11-ene-4α,5α-diol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 5α,7β,9α,10β,13α-Pentaacetoxy-2α- benzoyloxytax- |
C37H48O14 |
716.3044 |
A |
H |
OBz |
OH |
OAc |
OAc |
OAc |
OH |
172 |
T. mairei |
Liang and Kingston (1993) |
| 11-ene-4α,20-diol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxuspine R |
C32H47O14 |
654.2888 |
A |
OH |
OH |
H |
OAc |
OAc |
OAc |
OAc |
957 |
T. cuspidata |
Wang et al. (1996a) |
| Taxumairol O |
C28H42O13 |
586.2625 |
A |
OH |
OH |
OH |
OH |
OAc |
OAc |
OH |
168 |
T. mairei |
Shen et al. (2000) |
| 2,20-O-Diacetyltaxumairol N |
C32H46O15 |
670.2837 |
A |
OH |
OAc |
OH |
OH |
OAc |
OAc |
OAc |
3944 |
T. chinensis |
Xia et al. (2005a) |
| Wallitaxane G |
C31H40O12 |
604.2520 |
A |
OH |
OH |
OH |
OAc |
OH |
=O |
OBz |
3933 |
T. wallichiana |
Dang et al. (2017) |
| Wallitaxane H |
C29H36O11 |
560.2258 |
B |
OH |
OH |
OH |
OAc |
OH |
=O |
OBz |
3934 |
T. wallichiana |
Dang et al. (2017) |
| _______________________________________________________________________________________________________________________________________________________ |
Table 10. Group I.9. 6/8/6-Taxanes with C-12(17) or C-13(17)-oxido bridge.
_________________________________________________________________________________________________________________
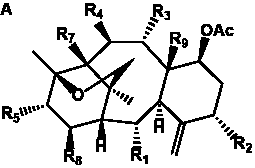
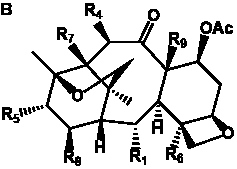
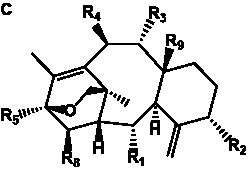
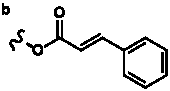
| Abbreviations: |
| n.a., not applicable |
| OAc, O-acetyl |
| OBz, O-benzoyl |
|
| Symbols: |
| =O, ketone functional group |
| ________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
R7 |
R8 |
R9 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-2 |
C-5 |
C-9 |
C-10 |
C-13 |
C-4 |
C-11 |
C-14 |
C-19 |
Record |
Source(s) |
|
| ________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 6/8/6-Taxanes with a C-12(17) oxido bridge and a C-4(20) double bond |
| 2α-Deacetyl-5α-decinnamoyltaxagifine |
C26H36O11 |
524.2258 |
A |
OH |
OH |
OAc |
OAc |
=O |
n.a. |
OH |
H |
CH3 |
141 |
T. chinensis |
Zhang and Jia (1991) |
| 5α-Decinnamoyltaxagifine |
C28H38O12 |
566.2363 |
A |
OAc |
OH |
OAc |
OAc |
=O |
n.a. |
OH |
H |
CH3 |
908 |
T. chinensis |
Zhang et al. (1990) |
| 5-Decinnamoyl-11-acetyl-19-hydroxyl- |
C30H40O14 |
624.2418 |
A |
OAc |
OH |
OAc |
OAc |
=O |
n.a. |
OAc |
H |
OH |
911 |
T. yunnanensis |
Chen et al. (1994a) |
| taxagifine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 19-Debenzoyl-19-acetyltaxinine M |
C30H40O14 |
624.2418 |
A |
OAc |
OH |
OAc |
OAc |
=O |
n.a. |
OH |
H |
OAc |
142 |
T. wallichiana |
Barboni et al. (1995b) |
| Taxinine M |
C35H42O14 |
686.2575 |
A |
OAc |
OH |
OAc |
OAc |
=O |
n.a. |
OH |
H |
OBz |
32 |
T. brevifolia |
Beutler et al. (1991) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
T. mairei |
Li and Shi (1999) |
| 5α-Acetyl-5α-decinnamoyltaxagifine |
C28H40O13 |
584.2469 |
A |
OAc |
OAc |
OAc |
OAc |
=O |
n.a. |
OH |
H |
CH3 |
909 |
T. chinensis |
Zhang et al. (1990) |
| Taxumairol R |
C37H44O15 |
728.2680 |
A |
OAc |
OAc |
OAc |
OAc |
=O |
n.a. |
OH |
H |
OBz |
138 |
T. mairei |
Shen et al. (2000b) |
| Taxezopidine N |
C39H51NO13 |
741.3360 |
A |
OAc |
a |
OAc |
OAc |
=O |
n.a. |
OH |
H |
CH3 |
140 |
T. cuspidata |
Morita et al. (2005) |
| 5α-[(R)-3'-Dimethylamino-3'-phenyl- |
C46H55NO15 |
861.3572 |
A |
OAc |
a |
OAc |
OAc |
=O |
n.a. |
OH |
H |
OBz |
139 |
T. wallichiana |
Prasain et al. (2001) |
| propanoyloxy]taxinine M |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxagifine |
C37H44O13 |
696.2782 |
A |
OAc |
b |
OAc |
OAc |
=O |
n.a. |
OH |
H |
CH3 |
1083 |
T. baccata |
Chauvière et al. (1982) |
| Taxuspine S |
C37H44O14 |
712.2731 |
A |
OAc |
b |
OAc |
OAc |
=O |
n.a. |
OH |
H |
OH |
144 |
T. cuspidata |
Wang et al. (1996a) |
| Taxuspine T |
C37H44O14 |
712.2731 |
A |
OH |
b |
OAc |
OH |
=O |
n.a. |
OH |
H |
OAc |
145 |
T. cuspidata |
Wang et al. (1996a) |
| 19-Acetoxytaxagifine |
C39H46O15 |
754.2837 |
A |
OAc |
b |
OAc |
OAc |
=O |
n.a. |
OH |
H |
OAc |
146 |
T. chinensis |
Fukushima et al. (1999) |
| Taxacin |
C44H48O15 |
816.2993 |
A |
OAc |
b |
OAc |
OAc |
=O |
n.a. |
OH |
H |
OBz |
1084 |
T. cuspidata |
Yoshizaki et al. (1988) |
| Tasumatrol L |
C36H44O15 |
716.2680 |
A |
OAc |
OH |
OAc |
OAc |
=O |
n.a. |
OH |
CH2OH |
OBz |
143 |
T. sumtrata |
Shen et al. (2005a) |
| 6/8/6-Taxanes with a C-12(17) oxido bridge and an oxetane ring |
| 4-Deacetyltaxagifine III |
C22H32O10 |
456.1995 |
B |
OH |
n.a. |
=O |
OH |
OH |
OH |
OH |
H |
CH3 |
148 |
T. chinensis |
Zhang and Jia (1991) |
| Taxagifine III |
C24H34O11 |
498.2101 |
B |
OH |
n.a. |
=O |
OH |
OH |
OAc |
OH |
H |
CH3 |
147 |
T. chinensis |
Zhang and Jia (1991) |
| 6/8/6-Taxanes with a C-13(17) oxido bridge and a C-4(20) double bond |
| Taxezopidine M |
C37H49NO10 |
667.3356 |
C |
OAc |
a |
OAc |
OAc |
OH |
n.a. |
n.a. |
H |
CH3 |
160 |
T. cuspidata |
Morita et al. (2005) |
| Taxezopidine A |
C26H36O9 |
492.2359 |
C |
OAc |
OH |
OAc |
OAc |
OH |
n.a. |
n.a. |
H |
CH3 |
161 |
T. cuspidata |
Wang et al. (1997) |
| Taxezopidine J |
C35H42O10 |
622.2778 |
C |
OAc |
b |
OAc |
OAc |
OH |
n.a. |
n.a. |
H |
CH3 |
162 |
T. cuspidata |
Shigemori et al. (1999) |
| ________________________________________________________________________________________________________________________________________________________________ |
Table 11. Group I.10. 6/8/6-Taxanes. Taxa-4(20),12-dienes.
_________________________________________________________________________________________________________________
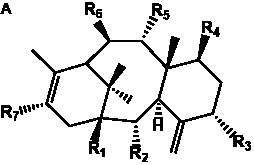
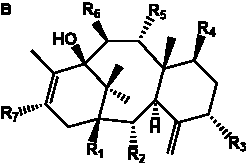
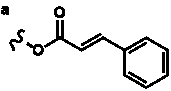
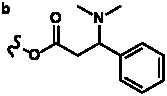
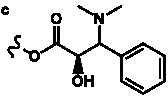
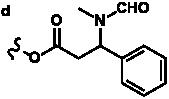
| ________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
R7 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-1 |
C-2 |
C-5 |
C-7 |
C-9 |
C-10 |
C-13 |
Record |
Source(s) |
|
| ________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,9α,7β,13-Tetraacetoxytaxa-4(20),12-diene- |
C28H40O11 |
552.2571 |
A |
H |
OAc |
OH |
OAc |
OAc |
OH |
OAc |
151 |
T. canadensis |
Shi et al. (2005a) |
| 5α,10β,11β-triol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 5-Decinnamoyltaxuspine D |
C30H42O12 |
594.2676 |
A |
H |
OAc |
OH |
OAc |
OAc |
OAc |
OAc |
149 |
T. canadensis |
Zamir et al. (1999a) |
| 7-Deacetoxytaxuspine D |
C37H46O12 |
682.2989 |
A |
H |
OAc |
a |
OH |
OAc |
OAc |
OAc |
150 |
T. canadensis |
Shi et al. (2003b) |
| Taxezopidine K |
C37H46O12 |
682.2989 |
A |
H |
OAc |
a |
OAc |
OAc |
OH |
OAc |
153 |
T. cuspidata |
Shigemori et al. (1999) |
| Taxuspine D |
C39H48O13 |
724.3095 |
A |
H |
OAc |
a |
OAc |
OAc |
OAc |
OAc |
3948 |
T. cuspidata |
Kobayashi et al. (1995b) |
| Taxuspine P |
C41H55NO13 |
769.3673 |
A |
H |
OAc |
b |
OAc |
OAc |
OAc |
OAc |
152 |
T. cuspidata |
Kobayashi et al. (1996) |
| 2α,10β,13α-Triacetoxy-5α-[3'-(N-formyl-N-methyl- |
C37H49NO10 |
667.3356 |
A |
H |
OAc |
d |
H |
OH |
OAc |
OAc |
3928 |
T. canadensis |
Zhang et al. (2013) |
| amino)-3'-phenylpropanoyloxy] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| taxa-4(20),12-dien-9α-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,7β,9α,10β,13-Pentaacetoxy-5α-(2'-hydroxy- |
C41H55NO14 |
785.3623 |
B |
H |
OAc |
c |
OAc |
OAc |
OAc |
OAc |
885 |
T. canadensis |
Zhang et al. (2008b) |
| 3'-N,N-dimethylamino-3'-phenylpropanoyloxy) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| taxa-4(20),12-dien-11β-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| ________________________________________________________________________________________________________________________________________________________ |
Table 12. Group I.11 6/8/6-Taxanes with a C-4(5)-double bond.
_________________________________________________________________________________________________________
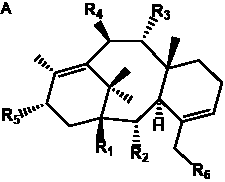
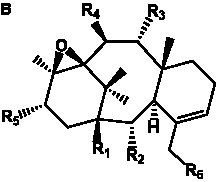
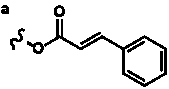
| Abbreviations: |
| OAc, O-acetyl |
|
| Symbols: |
| =O, ketone functional group |
| _________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-1 |
C-2 |
C-9 |
C-10 |
C-13 |
C-20 |
Record |
Source(s) |
|
| _________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Taxanes with C-4(5)- and C-11(12)-double bonds |
| 9α-Acetoxy-2α,20-dihydroxytaxa-4(5),11(12)-dien-13-one |
C22H32O5 |
376.2250 |
A |
H |
OH |
OAc |
H |
=O |
OH |
157 |
T. mairei |
Shi et al. (1999f) |
| 9α,10β-Diacetoxy-2α,20-dihydroxytaxa-4(5),11(12)-dien-13-one |
C24H34O7 |
434.2305 |
A |
H |
OH |
OAc |
OAc |
=O |
OH |
910 |
T. mairei |
Shi et al. (1999a) |
| 2α10β,13α-Triacetoxytaxa-4(5),11(12)-diene-9α,20-diol |
C26H38O8 |
478.2567 |
A |
H |
OAc |
OH |
OAc |
OAc |
OH |
159 |
T. canadensis |
Shi et al. (2003b) |
| 2α,10β,13α-Triacetoxy-20-cinnamoyloxytaxa-4 (5),11(12)-dien-9α-ol |
C35H44O9 |
608.2985 |
A |
H |
OAc |
OH |
OAc |
OAc |
a |
3865 |
T. canadensis |
Shi et al. (2003d) |
| 2α,9α,10β,13α-Tetraacetoxy-20-cinnamoyloxytaxa-4(5),11(12)-diene |
C37H46O10 |
650.3091 |
A |
H |
OAc |
OAc |
OAc |
OAc |
a |
158 |
T. canadensis |
Zhang et al. (2000a) |
| |
|
|
|
|
|
|
|
|
|
|
T. mairei |
Li et al. (2006) |
| Taxanes with a C-4(5)-double bond and C-11,12-epoxide |
| 2α,10β-Diacetoxy-11β,12β-epoxy-9α,20-dihydroxytaxa-4-en-13-one |
C24H34O8 |
450.2254 |
B |
H |
OAc |
OH |
OAc |
=O |
OH |
963 |
T. cuspidata |
Shi et al. (2006b) |
| 2α,9α-Diacetoxy-11β,12β-epoxy-10β,20-dihydroxytaxa-4-en-13-one |
C24H34O8 |
450.2254 |
B |
H |
OAc |
OAc |
OH |
=O |
OH |
962 |
T. cuspidata |
Shi et al. (2006b) |
| 2α,9α,10β-Triacetoxy-11β,12β-epoxy-20-hydroxytaxa-4-en-13-one |
C26H36O9 |
492.2359 |
B |
H |
OAc |
OAc |
OAc |
=O |
OH |
164 |
T. canadensis |
Petzke et al. (2004) |
| 2α,9α,10β-Triacetoxy-20-cinnamoyoloxy-11β,12β-epoxytaxa- |
C35H42O10 |
622.2778 |
B |
H |
OAc |
OAc |
OAc |
=O |
a |
163 |
T. canadensis |
Shi et al. (2003b) |
| 4-en-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
| _________________________________________________________________________________________________________________________________________________________________ |
Table 13. Group I.12. Miscellaneous 6/8/6-taxanes.
_________________________________________________________________________________________________________
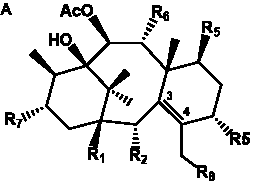
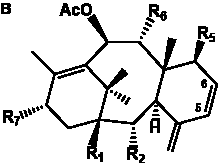
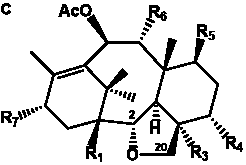
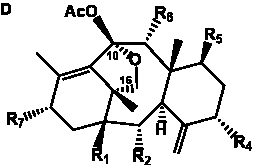
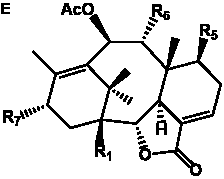
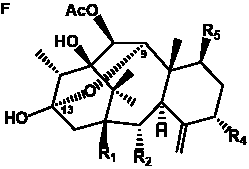
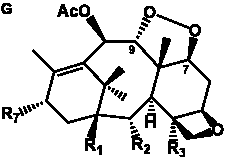
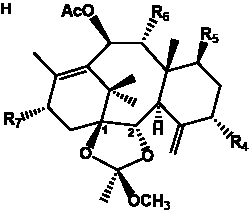
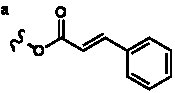
| Abbreviations: |
| n.a., not applicable |
| OAc, O-acetyl |
| OBz, O-benzoyl |
|
| Symbols: |
| =O, ketone functional group |
| ________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
R7 |
R8 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-1 |
C-2 |
C-4 |
C-5 |
C-7 |
C-9 |
C-13 |
C-20 |
Record |
Source(s) |
|
| ________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxezopidine B |
C26H38O10 |
510.2465 |
A |
H |
OAc |
n.a. |
OH |
H |
OAc |
=O |
OH |
784 |
T. cuspidata |
Wang et al. (1998) |
| 2α,10β,13α-Triacetoxytaxa-4(20),5,11-trien-9-ol |
C26H36O7 |
460.2461 |
B |
H |
OAc |
n.a. |
n.a. |
H |
OH |
OAc |
n.a. |
883 |
T. canadensis |
Shi et al. (2003a) |
| Taxuyunnanine Z |
C26H38O11 |
526.2414 |
C |
OH |
n.a. |
OH |
OAc |
OH |
OH |
OAc |
n.a. |
783 |
T. yunnanensis |
Li et al. (2003) |
| Taxuspine K |
C30H42O13 |
610.2625 |
C |
OH |
n.a. |
OAc |
OH |
OAc |
OAc |
OAc |
n.a. |
753 |
T. cuspidata |
Wang et al. (1996b) |
| Taxiwallinine |
C32H40O6 |
520.2824 |
D |
H |
H |
n.a. |
a |
H |
H |
OAc |
n.a. |
3974 |
T. mairei |
Lun etal. (2017) |
| Taxchinin N |
C30H38O12 |
590.2363 |
E |
OAc |
n.a. |
n.a. |
n.a. |
OAc |
OAc |
OAc |
n.a. |
16299 |
T. chinensis |
Zhao et al. (2006) |
| (12αH)-2α,10β-Diacetoxy-5α-cinnamoyloxy- |
C33H42O9 |
582.2829 |
F |
H |
OAc |
n.a. |
a |
H |
n.a. |
OH |
n.a. |
888 |
T. cuspidata |
Zhang et al. (2008) |
| 9α,13α-epoxytax-4(20)-ene-11β,13β-diol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 4α,10β,13α-Acetoxy-2α-benzoyloxy-7β,9α- |
C33H42O12 |
630.26763 |
G |
OH |
OBz |
OAc |
n.a. |
n.a. |
n.a. |
OAc |
n.a. |
3970 |
T. canadensis |
Pan et al. (2011) |
| epidioxy-5β,20-epoxytax-11-en-1β-ol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Baccataxinine |
C36H44O10 |
636.29345 |
H |
n.a. |
n.a. |
n.a. |
a |
H |
OAc |
=O |
n.a. |
3963 |
T. baccata |
Lei et al. (2018) |
| ________________________________________________________________________________________________________________________________________________________ |
Table 14. Group II.1. 11(15→1)Abeotaxanes with a C-4(20),11-double bond.
____________________________________________________________________________________________________
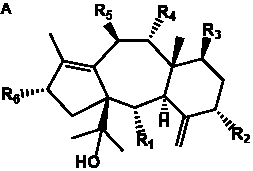
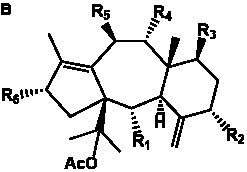
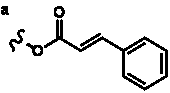
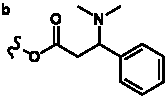
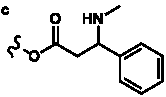
| Abbreviations: |
| OAc, O-acetyl |
| OBz, O-benzoyl |
| OGlc, O-β-D-glucopyranosyl |
| |
| Symbols: |
| =O, ketone functional group |
| ________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-2 |
C-5 |
C-7 |
C-9 |
C-10 |
C-13 |
Record |
Source(s) |
|
| ________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 11(15→1)Abeotaxadienes with no functional group at C-2 |
| 7-Debenzoyloxy-10-deacetylbrevifoliol |
C22H34O6 |
394.2355 |
A |
H |
OH |
H |
OAc |
OH |
OH |
914 |
T. wallichiana |
Appendino et al. (1993b) |
| 9α,13α-Diacetoxy-11(15→1)abeotaxa-4(20),11-diene |
C26H38O8 |
478.2567 |
A |
H |
OH |
H |
OAc |
OH |
OAc |
748 |
T. yunnanensis |
Shi et al. (1999g) |
| 5α,10β,15-triol |
|
|
|
|
|
|
|
|
|
|
|
|
| 9α,10β-Diacetoxy-11(15→1)abeotaxa-4(20),11-diene |
C24H36O7 |
436.2461 |
A |
H |
OH |
H |
OAc |
OAc |
OH |
950 |
T. mairei |
Shi et al. (1999b) |
| 5α,13α,15-triol |
|
|
|
|
|
|
|
|
|
|
|
|
| 9α,10β,13α-Triacetoxy-11(15→1)abeotaxa-4(20),11- |
C26H38O8 |
478.2567 |
A |
H |
OH |
H |
OAc |
OAc |
OAc |
951 |
T. mairei |
Shi et al. (1999b) |
| diene-5α,15-diol |
|
|
|
|
|
|
|
|
|
|
|
|
| 7β,9α-Diacetoxy-5α,15-dihydroxy-11(15→1)abeotaxa- |
C24H34O7 |
434.2305 |
A |
H |
OH |
OAc |
OAc |
H |
=O |
4138 |
T. mairei |
Huo et al. (2007c) |
| 4(20),11-dien-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
| Taxawallin F |
C26H38O9 |
494.2516 |
A |
H |
OH |
OAc |
OAc |
OH |
OAc |
4104 |
T. wallichiana |
Zhang et al. (1995b) |
| Taxawallin H |
C26H38O9 |
494.2516 |
A |
H |
OH |
OAc |
OAc |
OAc |
OH |
4106 |
T. wallichiana |
Zhang et al. (1995b) |
| 7β,9α,10β,13α-Tetraacetoxy-11(15→1)abeotaxa- |
C28H40O10 |
536.2621 |
A |
H |
OH |
OAc |
OAc |
OAc |
OAc |
4141 |
T. canadensis |
Petzke et al. (2004) |
| 4(20),11-diene-5α,15-diol |
|
|
|
|
|
|
|
|
|
|
|
|
| Brevifoliol |
C31H40O9 |
556.2672 |
A |
H |
OH |
OAc |
OAc |
OBz |
OH |
889 |
T. brevifolia |
Appendino et al. (1993b) |
| 13-Acetylbrevifoliol |
C33H42O10 |
598.2778 |
A |
H |
OH |
OAc |
OAc |
OBz |
OAc |
50 |
T. wallichiana |
Appendino et al. (1993b) |
| |
|
|
|
|
|
|
|
|
|
|
T. mairei |
Li et al. (2006) |
| 9-Deacetyl-9-benzoyl-10-debenzoylbrevifoliol |
C29H38O8 |
514.2567 |
A |
H |
OH |
OAc |
OBz |
OH |
OH |
751 |
T. brevifolia |
Chen and Kingston (1994) |
| Taxawallin D |
C33H42O10 |
598.2778 |
A |
H |
OH |
OAc |
OBz |
OAc |
OAc |
4136 |
T. wallichiana |
Zhang et al. (1995b) |
| 5α,9α,10β,13α-Tetracetoxy-11(15→1)abeotaxa-4(20),11- |
C28H40O9 |
520.2672 |
A |
H |
OAc |
H |
OAc |
OAc |
OAc |
799 |
T. wallichiana |
Choudhary et al. (2002) |
| dien-15-ol |
|
|
|
|
|
|
|
|
|
|
|
|
| 5,10,13-Triacetyl-10-debenzoylbrevifoliol |
C30H42O11 |
578.2727 |
A |
H |
OAc |
OAc |
OAc |
OAc |
OAc |
4125 |
T. wallichiana |
Chattopadhyay et al. (1999) |
| 13α-Acetoxy-5α-cinnamoyloxy-11(15→1)abeotaxa- |
C31H40O7 |
524.2774 |
A |
H |
a |
H |
OH |
OH |
OAc |
750 |
T. yunnanensis |
Kiyota et al. (2001) |
| 4(20),11-diene-9α,10β,15-triol |
|
|
|
|
|
|
|
|
|
|
|
|
| Chinentaxunine |
C33H42O8 |
566.2880 |
A |
H |
a |
H |
OAc |
OH |
OH |
1043 |
T. chinensis |
Shen et al. (1999a) |
| 9α,13α-Diacetoxy-5α-cinnamoyloxy-11(15→1) |
C33H42O8 |
566.2880 |
A |
H |
a |
H |
OAc |
OH |
OAc |
938 |
T. mairei |
Shi et al. (2000) |
| abeotaxa-4(20),11-diene-10β,15-diol |
|
|
|
|
|
|
|
|
|
|
|
|
| 7-Deacetoxytaxuspine J |
C35H44O9 |
608.2985 |
A |
H |
a |
H |
OAc |
OAc |
OAc |
746 |
T. cuspidata |
Murakami et al. (1999b) |
| 9α,13α-Diacetoxy-10β-benzoyloxy-5α-cinnamoyloxy- |
C40H46O9 |
670.3142 |
A |
H |
a |
H |
OAc |
OBz |
OAc |
747 |
T. yunnanensis |
Shi et al. (2000e) |
| 11(15→1)abeotaxa- 4(20),11-dien-15-ol |
|
|
|
|
|
|
|
|
|
|
|
|
| Taxuspine M |
C35H44O10 |
624.2934 |
A |
H |
a |
OAc |
OAc |
OH |
OAc |
752 |
T. cuspidata |
Wang et al. (1996b) |
| Taxuspine J |
C37H46O11 |
666.3040 |
A |
H |
a |
OAc |
OAc |
OAc |
OAc |
766 |
T. cuspidata |
Kobayashi et al. (1995a) |
| Taxchinin H |
C40H46O10 |
686.3091 |
A |
H |
a |
OAc |
OAc |
OBz |
OH |
755 |
T. chinensis |
Tanaka et al. (1994) |
| |
|
|
|
|
|
|
|
|
|
|
T. wallichiana |
Barboni et al. (1995b); |
| |
|
|
|
|
|
|
|
|
|
|
|
Zhang et al. (1995b) |
| Taxuspine A |
C42H48O11 |
728.3197 |
A |
H |
a |
OAc |
OAc |
OBz |
OAc |
4137 |
T. cuspidata |
Kobayashi et al. (1994) |
| |
|
|
|
|
|
|
|
|
|
|
T. brevifolia |
Appendino et al. (1993b) |
| 9α,13α-Diacetoxy-10β-benzoyloxy-5α-[(R)-3'- |
C42H53NO9 |
715.3720 |
A |
H |
b |
H |
OAc |
OBz |
OAc |
749 |
T. yunnanensis |
Shi et al. (1999g) |
| dimethylamino-3'-phenylpropanoyloxy]- |
|
|
|
|
|
|
|
|
|
|
|
|
| 11(15→1)abeotaxa-4(20),11-dien-15-ol |
|
|
|
|
|
|
|
|
|
|
|
|
| 7β,9α,13α-Triacetoxy-10β-benzoyloxy-5α-(3'-di- |
C42H51NO11 |
745.3462 |
A |
H |
b |
OAc |
OAc |
OBz |
OAc |
891 |
T. brevifolia |
Appendino et al. (1993b); |
| methylamino-3'-phenylpropanoyloxy)-11- |
|
|
|
|
|
|
|
|
|
|
|
Chu et al. (1994) |
| (15→1)abeotaxa-4(20),11-dien-1β-ol |
|
|
|
|
|
|
|
|
|
|
|
|
| 13α-acetoxy-5α-[(R)-3'-dimethylamino- |
C33H47NO7 |
569.3353 |
A |
H |
b |
H |
OH |
OH |
OAc |
942 |
T. yunnanensis |
Shi et al. (1999h) |
| 3'-phenylpropanoyloxy]-11(15→1)abeotaxa- |
|
|
|
|
|
|
|
|
|
|
|
|
| 4(20),11-diene-9α,10β-diol |
|
|
|
|
|
|
|
|
|
|
|
|
| 7β,9α,13α-Triacetoxy-10β-benzoyloxy-5α-(3'-methyl- |
C43H53NO11 |
759.3619 |
A |
H |
c |
OAc |
OAc |
OBz |
OAc |
890 |
T. brevifolia |
Appendino et al. (1993b); |
| amino-3'-phenylpropanoyloxy)-11- (15→1)abeotaxa- |
|
|
|
|
|
|
|
|
|
|
|
Chu et al. (1994) |
| 4(20),11-dien-1β-ol |
|
|
|
|
|
|
|
|
|
|
|
|
| 11(15→1)Abeotaxadienes with an acetyl functional group at C-2 |
| Teixidol |
C28H40O10 |
536.2621 |
A |
OAc |
OH |
H |
OAc |
OAc |
OAc |
774 |
T. baccata |
Soto et al. (1996) |
| Taxuspine Y |
C31H38O9 |
554.2516 |
A |
OAc |
OH |
H |
OAc |
OBz |
=O |
768 |
T. cuspidata |
Shigemori et al. (1997) |
| Taxacustone |
C24H34O8 |
450.2254 |
A |
OAc |
OH |
H |
OAc |
OH |
=O |
4146 |
T. cuspidata |
Tong et al. (1995) |
| 2α,9α,15-Triacetoxy-11(15→1)abeotaxa-4(20),11-diene- |
C26H38O10 |
510.2465 |
B |
OAc |
OH |
OH |
OAc |
OH |
OH |
4147 |
T. wallichiana |
Choudhary et al. (2002) |
| 5α,7β,10β,13α-tetraol |
|
|
|
|
|
|
|
|
|
|
|
|
| Taxuspine O |
C26H38O11 |
526.2414 |
A |
OAc |
OH |
OH |
OAc |
OAc |
=O |
767 |
T. cuspidata |
Kobayashi et al. (1996) |
| 2α-Acetoxy-9α-benzoyloxy-5α,7β,10β,15-tetrahydroxy- |
C29H36O9 |
528.2359 |
A |
OAc |
OH |
OH |
OBz |
OH |
=O |
794 |
T. yunnanensis |
Nguyen et al. (2003) |
| 11(15→1)abeotaxa-4(20),11-dien-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 10-Debenzoyl-2α-acetoxybrevifoliol |
C26H38O10 |
510.2465 |
A |
OAc |
OH |
OAc |
OAc |
OH |
OH |
4142 |
T. wallichiana |
Barboni et al. (1995b) |
| Taxchinin G |
C28H40O11 |
552.2571 |
A |
OAc |
OH |
OAc |
OAc |
OH |
OAc |
754 |
T. chinensis |
Li et al. (1993) |
| Taxchinin A |
C33H42O11 |
614.2727 |
A |
OAc |
OH |
OAc |
OAc |
OBz |
OH |
769 |
T. chinensis |
Fuji et al. (1992) |
| |
|
|
|
|
|
|
|
|
|
|
T. yunnanensis |
Liang et al. (2002) |
| Taxchinin D |
C35H44O12 |
656.2833 |
A |
OAc |
OH |
OAc |
OAc |
OBz |
OAc |
773 |
T. chinensis |
Li et al. (1993) |
| Taxuspinanane B |
C33H40O11 |
612.2571 |
A |
OAc |
OH |
OAc |
OAc |
OBz |
=O |
4144 |
T. cuspidata |
Morita et al. (1997b) |
| Taxchinin E |
C42H48O12 |
744.3146 |
A |
OAc |
OH |
OAc |
OAc |
OBz |
a |
756 |
T. chinensis |
Tanaka et al. (1994) |
| 9-Deacetyl-9-benzoyl-10-debenzoyltaxchinin A |
C31H40O10 |
572.2622 |
A |
OAc |
OH |
OAc |
OBz |
OH |
OH |
999 |
T. baccata |
Guo et al. (1996) |
| 13-Acetyl-9-deacetyl-9-benzoyl-10-debenzoyltaxchinin A |
C33H42O11 |
614.2727 |
A |
OAc |
OH |
OAc |
OBz |
OH |
OAc |
983 |
T. chinensis |
Shi et al. (1998b) |
| 5α-O-(β-D-Glucopyranosyl)-10β-benzoyltaxacustone |
C37H48O14 |
716.3044 |
A |
OAc |
OGlc |
H |
OAc |
OBz |
=O |
797 |
T. cuspidata |
Tong et al. (1995) |
| Taxamedin A |
C37H46O11 |
666.3040 |
A |
OAc |
a |
H |
OAc |
OAc |
OAc |
985 |
T. x media |
Gao et al. (1997) |
| 9-Deacetyl-9-benzoyl-10-debenzoyl-5-cinnamoyltaxchinin A |
C40H46O11 |
702.3040 |
A |
OAc |
a |
OAc |
OBz |
OH |
OH |
982 |
T. mairei |
Shi et al. (1998c) |
| (-)-2α-Acetoxy-2',7-dideacetoxy-1-hydroxy-11(15→1) |
C39H53NO11 |
711.3619 |
A |
OAc |
b |
H |
OAc |
OAc |
OAc |
4145 |
T. baccata |
Doss et al. (1997) |
| abeoaustrospicatine |
|
|
|
|
|
|
|
|
|
|
|
|
| 11(15→1)Abeotaxadienes with a benzoyl functional group at C-2 |
| 2-Deacetyl-2α-benzoyl-5,13-diacetyltaxchinin A |
C42H48O13 |
760.3095 |
A |
OBz |
OAc |
OAc |
OAc |
OBz |
OAc |
814 |
T. brevifolia |
Rao and Jochum (1998) |
| ________________________________________________________________________________________________________________________________________________________________ |
Table 15. Group II.2. 11(15→1)Abeotaxadienes with a C-4,20-epoxide ring.
____________________________________________________________________________________________________
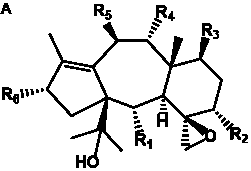
| Abbreviations: |
| OAc, O-acetyl |
| OBz, O-benzoyl |
| ____________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-2 |
C-5 |
C-7 |
C-9 |
C-10 |
C-13 |
Record |
Source(s) |
|
| _____________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,7β-Diacetoxy-9α-benzoyloxy-4β20-epoxy11(15→1)abeotax-11- |
C31H40O11 |
588.2571 |
A |
OAc |
OH |
OAc |
OBz |
OH |
OH |
917 |
T. mairei |
Shi et al. (1999i) |
| ene-5α,10β,13α,15-tetraol |
|
|
|
|
|
|
|
|
|
|
|
|
| Taxuchin A |
C32H44O14 |
652.2731 |
A |
OAc |
OAc |
OAc |
OAc |
OAc |
OAc |
843 |
T. chinensis |
Zhang et al. (1994d) |
| _____________________________________________________________________________________________________________________________________________________________ |
Table 16. Group II.3. 11(15→1)Abeotaxadienes with an oxetane ring.
____________________________________________________________________________________________________
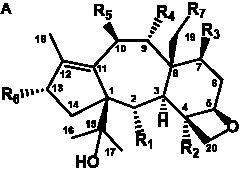
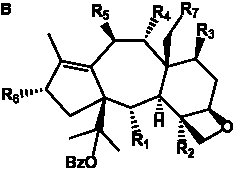
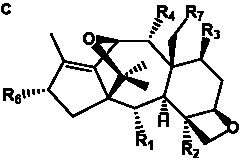
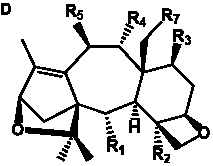
| Abbreviations: |
| OAc, O-acetyl |
| OBz, O-benzoyl |
| |
| Symbols: |
| =O, ketone functional group |
| _________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
R7 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-2 |
C-4 |
C-7 |
C-9 |
C-10 |
C-13 |
C-19 |
Record |
Source(s) |
|
| _________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 11(15→1)Abeotaxanes with oxetane ring and a hydroxyl group at C-13 |
| 4α,7β-Diacetoxy-9α-benzoyloxy-5β,20-epoxy- |
C31H41O11 |
589.2649 |
A |
OH |
OAc |
OAc |
OBz |
OH |
OH |
H |
986 |
T. mairei |
Shi et al. (1999j) |
| 11(15→1)abeotax-11-ene-2α,10β,13α,15-tetraol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxuyunnanine M (taxumairol Q) |
C24H36O10 |
484.2308 |
A |
OAc |
OAc |
OH |
OH |
OH |
OH |
H |
1016 |
T. yunnanensis |
Li et al. (2000) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. sumatrana |
Shen et al. (2002a) |
| 7,9-Dideacetyltaxayuntin |
C31H40O11 |
588.2571 |
A |
OAc |
OAc |
OH |
OH |
OBz |
OH |
H |
775 |
T. yunnanensis |
Zhong et al. (1996) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. brevifolia |
Rao et al. (1996a) |
| Taxumairol K |
C31H40O11 |
588.2571 |
A |
OAc |
OAc |
OH |
OBz |
OH |
OH |
H |
1026 |
T. mairei |
Shen et al. (1998a) |
| Tasumatrol B |
C26H38O11 |
526.2414 |
A |
OAc |
OAc |
OAc |
OH |
OH |
OH |
H |
1013 |
T. sumatrana |
Shen et al. (2003) |
| Taxumairol W |
C28H40O12 |
568.2520 |
A |
OAc |
OAc |
OAc |
OH |
OAc |
OH |
H |
1022 |
T. mairei |
Shen et al. (2001a) |
| 13-Decinnamoyl-9-deacetyltaxchinin B |
C33H42O12 |
630.2676 |
A |
OAc |
OAc |
OAc |
OH |
OBz |
OH |
H |
826 |
T. wallichiana |
Chattopadhyay et al. (1996) |
| Taxacustin (10,13-deacetylabeobaccatin IV) |
C28H40O12 |
568.2520 |
A |
OAc |
OAc |
OAc |
OAc |
OH |
OH |
H |
824 |
T. cuspidata |
Tong et al. (1993) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. wallichiana |
Chattopadhyay et al. (1995) |
| Taxayuntin F (taxchinin L) |
C33H42O12 |
630.2676 |
A |
OAc |
OAc |
OAc |
OBz |
OH |
OH |
H |
777 |
T. yunnanensis |
Yue et al. (1995a) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. chinensis |
Tanaka et al. (1996) |
| Taxayuntin H |
C30H42O13 |
610.2625 |
A |
OAc |
OAc |
OAc |
OAc |
OAc |
OH |
H |
778 |
T. yunnanensis |
Zhou et al. (1998) |
| 13-Deacetylbaccatin VI (taxayuntin; |
C35H44O13 |
672.2782 |
A |
OAc |
OAc |
OAc |
OAc |
OBz |
OH |
H |
873 |
T. wallichiana |
Appendino et al. (1993b) |
| taxayunnansin A) |
|
|
|
|
|
|
|
|
|
|
|
|
incorrect structure reported |
| |
|
|
|
|
|
|
|
|
|
|
|
|
by Barboni et al. (1993) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. yunnanensis |
Zhang et al. (1995c) |
| Taxuspine Q |
C33H46O13 |
650.2938 |
A |
OAc |
OAc |
OAc |
OAc |
a |
OH |
H |
764 |
T. cuspidata |
Wang et al. (1996a) |
| 4-Deacetyl-11(15→1)abeobaccatin VI |
C35H44O13 |
672.2782 |
A |
OBz |
OH |
OAc |
OAc |
OAc |
OAc |
H |
836 |
T. media |
Barboni et al. (1994b) |
| 7,13-Dideacetyl-9,10-debenzoyltaxchinin C |
C29H38O10 |
546.2465 |
A |
OBz |
OAc |
OH |
OH |
OH |
OH |
H |
820 |
T. brevifolia |
Chen and Kingston (1994) |
| Taxumairol Z |
C33H42O13 |
646.2625 |
A |
OBz |
OAc |
OH |
OH |
OH |
OAc |
OAc |
1010 |
T. mairei |
Shen et al. (2002c) |
| Tasumatrol O |
C31H38O12 |
602.2363 |
A |
OBz |
OAc |
OH |
=O |
OAc |
OH |
OH |
13083 |
T. sumatrana |
Shen et al. (2005b) |
| Taxuspinanane F (9-deacetyltaxayuntin E) |
C31H40O11 |
588.2571 |
A |
OBz |
OAc |
OAc |
OH |
OH |
OH |
H |
4012 |
T. cuspidata |
Morita et al. (1998b) |
| (4α,7β-diacetoxy-2α-benzoyloxy-5β,20-epoxy- |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 1β,9α,10β,13α,15-pentahydroxy- |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 11(15→1)abeotax-11-ene) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxayuntin E |
C33H42O12 |
630.2676 |
A |
OBz |
OAc |
OAc |
OAc |
OH |
OH |
H |
776 |
T. yunnanensis |
Yue et al. (1995a) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. mairei |
Li et al. (2006) |
| Taxayuntin A |
C36H42O11 |
650.2727 |
A |
OBz |
OAc |
OBz |
OH |
OH |
OH |
H |
872 |
T. yunnanensis |
Chen et al. (1993b) |
| 15-O-Benzoyl-10-deacetyl-2-debenzoyl- |
C29H34O10 |
542.2152 |
B |
OH |
OAc |
OH |
=O |
=O |
OH |
H |
834 |
T. canadensis |
Zhang et al. (2000b) |
| 10-dehydro-abeobaccatin III |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 11(15→1)Abeotaxanes with oxetane ring and an acetyl group at C-13 |
| Taxuyunnanine F |
C30H42O13 |
610.2625 |
A |
OAc |
OAc |
OAc |
OAc |
OH |
OAc |
H |
1031 |
T. yunnanensis |
Zhang et al. (1995e) |
| Taxchinin M |
C35H44O13 |
672.2782 |
A |
OAc |
OAc |
OAc |
OBz |
OH |
OAc |
H |
763 |
T. mairei |
Tanaka et al. (1996) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. floridana |
Rao and Johnson (1998) |
| Taxchinin J |
C42H48O13 |
760.3095 |
A |
OAc |
OAc |
OAc |
OBz |
OH |
b |
H |
759 |
T. chinensis |
Tanaka et al. (1994) |
| 13-Acetyl-13-decinnamoyltaxchinin B |
C37H46O14 |
714.2888 |
A |
OAc |
OAc |
OAc |
OAc |
OBz |
OAc |
H |
828 |
T. baccata |
Das et al. (1995) |
| Taxchinin B |
C44H50O14 |
802.3201 |
A |
OAc |
OAc |
OAc |
OAc |
OBz |
b |
H |
770 |
T. chinensis |
Fuji et al. (1993) |
| Taxchinin K |
C42H48O14 |
776.3044 |
A |
OAc |
OAc |
OAc |
OBz |
OBz |
OAc |
H |
757 |
T. chinensis |
Tanaka et al. (1994) |
| Taxayuntin B (2α-debenzoyl-2α-acetyltaxayuntin A) |
C31H40O11 |
588.2571 |
A |
OAc |
OAc |
OBz |
OH |
OH |
OH |
H |
762 |
T. yunnanensis |
Chen et al. (1993b) |
| (2α,4α-diacetoxy-7β-benzoyloxy-5β,20-epoxy- |
|
|
|
|
|
|
|
|
|
|
|
T. mairei |
Shi et al. (1999i) |
| 1β,9α,10β,13α,15-pentahydroxy- |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 11(15→1)abeotax-11-ene) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 7,9,10-Trideacetylabeobaccatin VI |
C31H40O11 |
588.2571 |
A |
OBz |
OAc |
OH |
OH |
OH |
OAc |
H |
827 |
T. baccata |
Appendino et al. (1994a) |
| 7-deacetyltaxayuntin D |
C45H48O13 |
796.3095 |
A |
OBz |
OAc |
OH |
OBz |
OBz |
OAc |
H |
817 |
T. brevifolia |
Rao and Juchum (1998) |
| Taxayuntin C |
C42H48O14 |
776.3044 |
A |
OBz |
OAc |
OAc |
OAc |
OBz |
OAc |
H |
3989 |
T. yunnanensis |
Chen et al. (1993b) |
| 4α,7β-Diacetoxy-2α,9α-dibenzoyloxy-5β,20-epoxy- |
C38H44O12 |
692.2833 |
A |
OBz |
OAc |
OAc |
OBz |
OH |
OH |
H |
874 |
T. baccata |
Guo et al. (1995b) |
| 11(15→1)abeotax-11-ene-1β,10β,13α,15-tetraol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxchinin I |
C40H46O13 |
734.2938 |
A |
OBz |
OAc |
OAc |
OBz |
OH |
OAc |
H |
829 |
T. media |
Barboni et al. (1994b) |
| Taxayuntin D (taxchinin C; 9-deacetyl-9-benzoyl- |
C47H50O14 |
838.3201 |
A |
OBz |
OAc |
OAc |
OBz |
OBz |
OAc |
H |
771 |
T. chinensis |
Fuji et al. (1993) |
| taxayuntin C) |
|
|
|
|
|
|
|
|
|
|
|
T. yunnanensis |
Chen et al. (1993b) |
| |
|
|
|
|
|
|
|
|
|
|
|
T. brevifolia |
Huang et al. (1998); |
| |
|
|
|
|
|
|
|
|
|
|
|
|
incorrect structure published by |
| |
|
|
|
|
|
|
|
|
|
|
|
|
Chu et al. (1992) |
| 2,7-Dideacetyl-2,7-dibenzoyltaxayunnanine F |
C42H48O14 |
776.3044 |
A |
OBz |
OAc |
OBz |
OAc |
OH |
OAc |
H |
3973 |
T. brevifolia |
Rao and Juchum (1998) |
| 4α,9α,10β,13α-Tetraacetoxy-2α,7β-dibenzoyloxy- |
C42H48O14 |
776.3044 |
A |
OBz |
OAc |
OBz |
OAc |
OAc |
OAc |
H |
209 |
T. brevifolia |
Huang et al. (1998); |
| 5β,20-epoxy-11(15→1)abeotax-11-en-1β-ol |
|
|
|
|
|
|
|
|
|
|
|
|
incorrect structure published by |
| |
|
|
|
|
|
|
|
|
|
|
|
|
Chu et al. (1992) |
| 7-Deacetyl-7-benzoyltaxayuntin C |
C47H50O14 |
838.3201 |
A |
OBz |
OAc |
OBz |
OAc |
OBz |
OAc |
H |
819 |
T. brevifolia |
Rao and Juchum (1998) |
| 7-Deacetyl-7-benzoyltaxchinin I |
C45H48O13 |
796.3095 |
A |
OBz |
OAc |
OBz |
OBz |
OH |
OAc |
H |
818 |
T. brevifolia |
Rao and Juchum (1998) |
| 7,9-Di-O-acetyl-15-O-benzoyl-2-de-O-benzoyl- |
C33H42O12 |
630.2676 |
B |
OH |
OAc |
OH |
OH |
OAc |
OAc |
OBz |
835 |
T. canadensis |
Zhang et al. (2000b) |
| abeobaccatin VI |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 11(15→1)Abeotaxanes with oxetane ring and additional C-10,15- or C-13,15 ether ring. |
| 10,15-Epoxy-11(15→1)abeo- |
C29H34O9 |
526.2203 |
C |
OBz |
OAc |
OH |
=O |
n.a. |
OH |
|
887 |
T. wallichiana |
Appendino et al. (1993e) |
| 10-deacetylbaccatin III |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 13-Deacetoxy-13,15-epoxy-11(15→1)abeo- |
C35H42O12 |
654.2676 |
D |
OBz |
OAc |
OAc |
OAc |
OAc |
n.a. |
|
850 |
T. x media |
Barboni et al. (1994b) |
| 13-epi-baccatin VI |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 13,15-Epoxy-13-epi-taxayunnasin A |
C35H42O12 |
654.2676 |
D |
OAc |
OAc |
OAc |
OAc |
OBz |
n.a. |
|
16298 |
T. chinensis |
Zhao et al. (2006) |
| _________________________________________________________________________________________________________________________________________________________________ |
Table 17. Group II.4. 11(15→1)Abeotaxadienes with an opened oxetane ring.
____________________________________________________________________________________________________
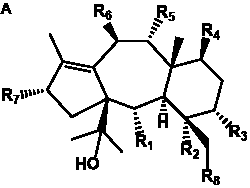
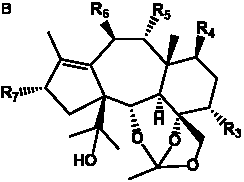
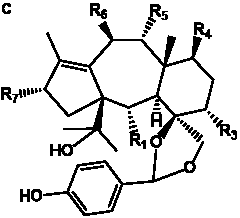
| Abbreviations: |
| OAc, O-acetyl |
| OBz, O-benzoyl |
| ________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
R7 |
R8 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-2 |
C-4 |
C-5 |
C-7 |
C-9 |
C-10 |
C-13 |
C-20 |
Record |
Source(s) |
|
| ________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxuyunnanine P |
C24H38O10 |
486.2465 |
A |
OH |
H |
OH |
OAc |
OAc |
OH |
OH |
OH |
6937 |
T. yunnanensis |
Li et al. (2001) |
| Taxuyunnanine Q |
C26H40O11 |
528.2571 |
A |
OH |
H |
OH |
OAc |
OAc |
OAc |
OH |
OH |
6938 |
T. yunnanensis |
Li et al. (2001) |
| Taxuyunnanine N |
C26H40O11 |
528.2571 |
A |
OH |
H |
OAc |
OAc |
OAc |
OH |
OH |
OH |
1017 |
T. yunnanensis |
Li et al. (2000) |
| Taxumairol V |
C28H42O12 |
570.2676 |
A |
OH |
H |
OAc |
OAc |
OAc |
OH |
OH |
OAc |
1021 |
T. mairei |
Shen et al. (2001a) |
| Taxuyunnanine V |
C28H42O12 |
570.2676 |
A |
OH |
H |
OAc |
OAc |
OAc |
OH |
OAc |
OH |
878 |
T. yunnanensis |
Li et al. (2002) |
| Taxumairol U |
C30H44O13 |
612.2782 |
A |
OH |
H |
OAc |
OAc |
OAc |
OH |
OAc |
OAc |
879 |
T. mairei |
Shen et al. (2001a) |
| Taxuyunnanine O |
C28H42O12 |
570.2676 |
A |
OH |
H |
OAc |
OAc |
OAc |
OAc |
OH |
OH |
1018 |
T. yunnanensis |
Li et al. (2000) |
| Taxayuntin J |
C30H44O13 |
612.2782 |
A |
OH |
H |
OAc |
OAc |
OAc |
OAc |
OH |
OAc |
779 |
T. yunnanensis |
Zhou et al. (1998) |
| Tasumatrol F |
C33H44O12 |
632.2833 |
A |
OH |
H |
OAc |
OAc |
OBz |
OH |
OH |
OAc |
1024 |
T. sumatrana |
Shen et al. (2005c) |
| Taxayuntin G |
C28H42O12 |
570.2676 |
A |
OAc |
H |
OAc |
OAc |
OAc |
OH |
OH |
OH |
6936 |
T. yunnanensis |
Yue et al. (1995b) |
| Taxumain A |
C30H44O13 |
612.2782 |
A |
OAc |
H |
OAc |
OAc |
OAc |
OH |
OH |
OAc |
1037 |
T. mairei |
Yang et al. (1999) |
| Taxumain B |
C32H46O14 |
654.2888 |
A |
OAc |
H |
OAc |
OAc |
OAc |
OH |
OAc |
OAc |
787 |
T. mairei |
Yang et al. (1999) |
| Taxuyunnanine S |
C20H34O9 |
418.2203 |
A |
OH |
OH |
OH |
OH |
OH |
OH |
OH |
OH |
875 |
T. yunnanensis |
Li et al. (2002) |
| Taxuyunnanine R |
C27H37O10 |
521.2387 |
A |
OH |
OH |
OH |
OH |
OH |
OH |
OH |
OBz |
6939 |
T. yunnanensis |
Li et al. (2001) |
| Taxuyunnanine T |
C24H38O11 |
502.2414 |
A |
OH |
OH |
OAc |
OH |
OH |
OH |
OH |
OAc |
876 |
T. yunnanensis |
Li et al. (2002) |
| Taxuyunnanine K |
C29H40O11 |
564.2571 |
A |
OH |
OH |
OAc |
OH |
OH |
OH |
OH |
OBz |
1014 |
T. yunnanensis |
Li et al. (2000) |
| Taxuyunnanine U |
C31H42O12 |
606.2676 |
A |
OH |
OH |
OAc |
OH |
OH |
OBz |
OH |
OAc |
877 |
T. yunnanensis |
Li et al. (2002) |
| Yunantaxusin A |
C35H46O14 |
690.2888 |
A |
OH |
OH |
OAc |
OAc |
OAc |
OBz |
OH |
OAc |
788 |
T. yunnanensis |
Zhang et al. (1994e) |
| Tasumatrol E |
C33H44O13 |
648.2782 |
A |
OH |
OH |
OAc |
OAc |
OBz |
OH |
OH |
OAc |
1023 |
T. sumatrana |
Shen et al. (2005c) |
| Taxuchin B |
C31H40O11Cl |
623.2259 |
A |
OAc |
OH |
OH |
OAc |
OBz |
OH |
OH |
Cl |
3980 |
T. chinensis |
Fang et al. (1997) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
T. yunnanensis |
Li et al. (2003) |
| Taxuyunnanine L |
C29H40O11 |
564.2571 |
A |
OBz |
OH |
OH |
OH |
OH |
OH |
OH |
OAc |
1015 |
T. yunnanensis |
Li et al. (2000a) |
| Taxuyunnanine X |
C31H40O11 |
588.2571 |
B |
n.a. |
n.a. |
OH |
OAc |
OBz |
OH |
OH |
n.a. |
781 |
T. yunnanensis |
Li et al. (2003) |
| Taxuyunnanine W |
C33H42O12 |
630.2676 |
B |
n.a. |
n.a. |
OAc |
OAc |
OBz |
OH |
OH |
n.a. |
780 |
T. yunnanensis |
Li et al. (2003) |
| 5-O-Acetyl-20-O-deacetyl-4,20-(p-hydroxyl- |
C36H44O12 |
668.2833 |
C |
OBz |
n.a. |
OAc |
OH |
OH |
OH |
OH |
n.a. |
871 |
T. chinensis |
Xia et al. (2005b) |
| benzylidenedioxy)-taxuyunnanine L |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| _______________________________________________________________________________________________________________________________________________________ |
Table 18. Group II.5. 11(15→1)Abeotaxanes with a C-2,20 ether ring.
____________________________________________________________________________________________________
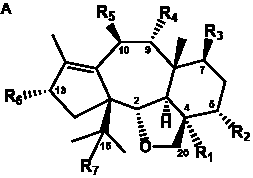
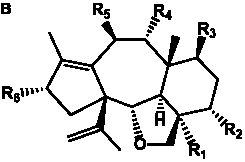
| Abbreviations: |
| OAc, O-acetyl |
| OBz, O-benzoyl |
| ______________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
R7 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-4 |
C-5 |
C-7 |
C-9 |
C-10 |
C-13 |
C-15 |
Record |
Source(s) |
|
| ______________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 1(15→1)Abeotaxanes with a C-2,20 ether ring and a substituent at C-15. |
| Taxumairol X |
C24H36O10 |
484.2308 |
A |
OH |
OH |
OAc |
OAc |
OH |
OH |
OH |
1008 |
T. mairei |
Shen et al. (2002c) |
| Taxumairol Y |
C26H38O11 |
526.2414 |
A |
OH |
OH |
OAc |
OAc |
OH |
OAc |
OH |
1009 |
T. mairei |
Shen et al. (2002c) |
| Taxumairol H |
C33H42O12 |
630.2676 |
A |
OH |
OH |
OAc |
OAc |
OAc |
OH |
OBz |
1002 |
T. mairei |
Shen et al. (2002a) |
| 10-O-Benzoyl-15-O-acetyltaxumairol X |
C33H42O12 |
630.2676 |
A |
OH |
OH |
OAc |
OAc |
OBz |
OH |
OAc |
842 |
T. chinensis |
Xia et al. (2005b) |
| Taxuyunnanine Y |
C29H38O10 |
546.2465 |
A |
OH |
OH |
OAc |
OBz |
OH |
OH |
OH |
782 |
T. yunnanensis |
Li et al. (2003) |
| 4α,10β,13α-Triacetoxy-15-benzoyloxy-2α,20β-epoxy- |
C33H42O12 |
630.2676 |
A |
OAc |
OH |
OH |
OH |
OAc |
OAc |
OBz |
844 |
T. canadensis |
Zhang et al. (2009) |
| 11(15→1)abeotax-11-ene-5α,7β,9α-triol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxumairol G |
C37H46O14 |
714.2888 |
A |
OAc |
OH |
OAc |
OAc |
OAc |
OAc |
OBz |
1001 |
T. mairei |
Shen et al. (2002a) |
| 4α,7β,9α,10β,15-Pentaacetoxy-2α,20β-epoxy- |
C30H42O13 |
610.2625 |
A |
OAc |
OH |
OAc |
OAc |
OAc |
OH |
OAc |
892 |
T. canadensis |
Zhang et al. (2009) |
| 11(15→1)abeotax-11-ene-5α,13α-diol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Taxuyunnanine E |
C33H42O12 |
630.2676 |
A |
OAc |
OH |
OAc |
OBz |
OH |
OAc |
OH |
918 |
T. mairei |
Zhang et al. (1995e) |
| Tasumatrol G |
C33H42O12 |
630.2676 |
A |
OBz |
OAc |
OAc |
OAc |
OH |
OH |
OH |
1025 |
T. sumatrana |
Shen et al. (2005c) |
| 1(15→1)Abeotaxanes with a C-2,20 ether ring and a C-15,16 double bond. |
| Taxumairol J |
C26H36O10 |
508.2308 |
B |
OH |
OH |
OAc |
OAc |
OAc |
OH |
n.a. |
1004 |
T. mairei |
Shen et al. (2002a) |
| Taxumairol I |
C28H38O11 |
550.2414 |
B |
OH |
OH |
OAc |
OAc |
OAc |
OAc |
n.a. |
1003 |
T. mairei |
Shen et al. (2002a) |
| 4α,13α-Diacetoxy-2α,20-epoxy-11(15→1)abeotaxa-11,15-diene- |
C24H34O9 |
466.2203 |
B |
OAc |
OH |
OH |
OH |
OH |
OAc |
n.a. |
847 |
T. canadensis |
Shi et al. (2007) |
| 5α,7β,9α,10β-tetraol |
|
|
|
|
|
|
|
|
|
|
|
|
|
| ______________________________________________________________________________________________________________________________________________________________________ |
Table 19. Group III. 11(15→1),11(10→9)Diabeotaxanes.
____________________________________________________________________________________________________
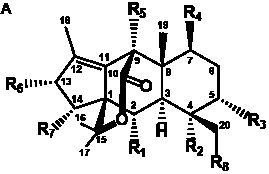
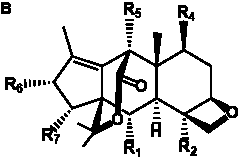
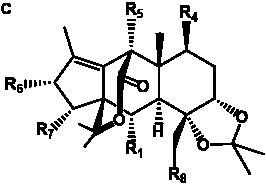
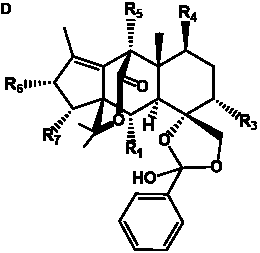
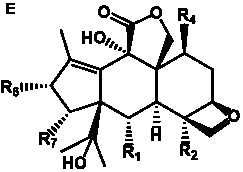
| Abbreviations: |
| OAc, O-acetyl |
| OBz, O-benzoyl |
| n.a., not applicable |
| ___________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
R7 |
R8 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-2 |
C-4 |
C-5 |
C-7 |
C-9 |
C-13 |
C-14 |
C-20 |
Record |
Source(s) |
|
| ___________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Tasumatrol T |
C27H34O9 |
502.2203 |
A |
H,H |
OH |
OH |
OH |
OH |
H,H |
OH |
OBz |
856 |
T. sumatrana |
Shen et al. (2007) |
| Tasumatrol P |
C29H36O10 |
544.2308 |
A |
H,H |
OH |
OAc |
OH |
OH |
H,H |
OH |
OBz |
852 |
T. sumatrana |
Shen et al. (2007) |
| Tasumatrol R |
C27H34O9 |
502.2203 |
A |
OBz |
OH |
OH |
OH |
OH |
H,H |
H,H |
OH |
854 |
T. sumatrana |
Shen et al. (2007) |
| Tasumatrol I |
C29H36O10 |
544.2308 |
A |
OBz |
OH |
OH |
OH |
OH |
H,H |
H,H |
OAc |
790 |
T. sumatrana |
Shen et al. (2005a) |
| 20-Acetoxy-2α-benzoyloxy-4α,5α,7β,9α,13α-pentahydroxy- |
C29H36O11 |
560.2258 |
A |
OBz |
OH |
OH |
OH |
OH |
OH |
H,H |
OAc |
851 |
T. yunnanensis |
Kiyota et al. (2001) |
| 11(15→1),11(10→9)bisabeotax-11-ene-10,15-lactone |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Tasumatrol H |
C29H36O11 |
560.2258 |
A |
OBz |
OH |
OAc |
OH |
OH |
OH |
H,H |
OH |
789 |
T. sumatrana |
Shen et al. (2005a) |
| Tasumatrol J |
C29H34O9 |
526.2203 |
B |
OBz |
OAc |
n.a. |
OH |
OH |
H,H |
H,H |
n.a. |
791 |
T. sumatrana |
Shen et al. (2005a) |
| Wallifoliol |
C29H34O10 |
542.2152 |
B |
OBz |
OAc |
n.a. |
OH |
OH |
OH |
H,H |
n.a. |
857 |
T. wallichiana |
Velde et al. (1994) |
| 13-O-Acetylwallifoliol |
C33H36O11 |
608.2258 |
B |
OBz |
OAc |
n.a. |
OH |
OH |
OAc |
H,H |
n.a. |
13053 |
T. sumatrana |
Shen et al. (2002b) |
| 13-oxo-Wollifoliane-10,15-olid |
C29H32O10 |
540.1995 |
B |
OBz |
OAc |
n.a. |
OH |
OH |
=O |
H,H |
n.a. |
|
T. baccata |
Ding et al. (2020) |
| Tasumatrol Q |
C32H40O11 |
600.2571 |
C |
OBz |
n.a. |
n.a. |
OH |
OH |
OH |
H,H |
OAc |
853 |
T. sumatrana |
Shen et al. (2007) |
| Tasumatrol S |
C29H36O10 |
544.2308 |
D |
H,H |
n.a. |
OAc |
OH |
OH |
H,H |
OH |
n.a. |
855 |
T. sumatrana |
Shen et al. (2007) |
| Tasumatrol A |
C29H34O11 |
558.2101 |
E |
OBz |
OAc |
n.a. |
OH |
OH |
OH |
H,H |
n.a. |
1012 |
T. sumatrana |
Shen et al. (2003) |
| ___________________________________________________________________________________________________________________________________________________________________ |
Table 20. Group IV. 2(3→20)Abeotaxanes.
____________________________________________________________________________________________________
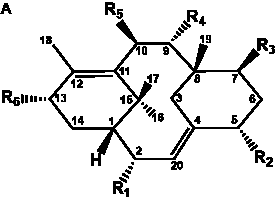
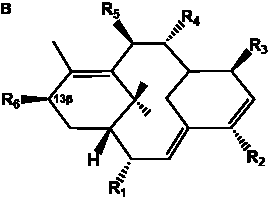
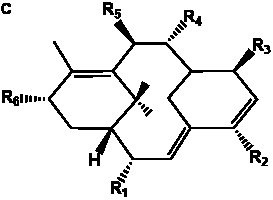
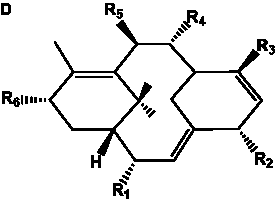
| __________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-2 |
C-5 |
C-7 |
C-9 |
C-10 |
C-13 |
Record |
Source(s) |
|
| __________________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 7β,13α-Diacetoxy-2α,5α,10β-trihydroxy-2(3→20)abeotaxa- |
C24H34O8 |
450.2254 |
A |
OH |
OH |
OAc |
=O |
OH |
OAc |
1041 |
T. mairei |
Shi et al. (1998d); |
| 4(20),11-dien-9-one |
|
|
|
|
|
|
|
|
|
|
|
Shi et al. (1999k) |
| 7β,10β-Diacetoxy-2α,5α,13α-trihydroxy-2(3→20)abeotaxa- |
C24H34O8 |
450.2254 |
A |
OH |
OH |
OAc |
=O |
OAc |
OH |
867 |
T. mairei |
Shi et al. (2006c) |
| 4(20),11-dien-9-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 2-Deacetyltaxin B |
C26H36O9 |
492.2359 |
A |
OH |
OH |
OAc |
=O |
OAc |
OAc |
4233 |
T. yunnanensis |
Yue et al. (1995c) |
| Taxezopidine P |
C31H38O8 |
538.2567 |
A |
OH |
a |
OH |
=O |
OH |
OAc |
954 |
T. cuspidata |
Ishiyama et al. (2007) |
| 10β,13α-Diacetoxy-5α-cinnamoyloxy-2α,7β-dihydroxy- |
C33H40O9 |
580.2672 |
A |
OH |
a |
OH |
=O |
OAc |
OAc |
861 |
T. yunnanensis |
Shi et al. (1999f) |
| 2(3→20)-abeotaxa-4(20),11-dien-9-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 2-Decacetyltaxuspine B |
C33H40O9 |
580.2672 |
A |
OH |
a |
OAc |
=O |
OH |
OAc |
926 |
T. mairei |
Oritani and Sugiyama |
| |
|
|
|
|
|
|
|
|
|
|
|
(1999) |
| 7β,10β,13α-Triacetoxy-5α-cinnamoyloxy-2α-hydroxy- |
C35H42O10 |
622.2778 |
A |
OH |
a |
OAc |
=O |
OAc |
OAc |
860 |
T. yunnanensis |
Shi et al. (1999f) |
| 2(3→20)abeotaxa-4(20),11-dien-9-one |
|
|
|
|
|
|
|
|
|
|
|
|
| Taxine C (2-deacetlytaxine A) |
C33H45NO9 |
599.3094 |
A |
OH |
c |
OH |
=O |
OH |
OAc |
4294 |
T. baccata |
Poupat et al. (1994) |
| 7β,10β,13α-Triacetoxy-5α-(3'-dimethylamino-3'-phenylpropanoyl- |
C37H49NO10 |
667.3356 |
A |
OH |
c |
OAc |
=O |
OAc |
OAc |
4302 |
T. canadensis |
Shi et al. (2006d) |
| oxy)-2'-hydroxy-2(3→20)abeotaxa-4(20),11-dien-9-one |
|
|
|
|
|
|
|
|
|
|
|
|
| Deaminoacyltaxine A |
C24H34O8 |
450.2254 |
A |
OAc |
OH |
OH |
=O |
OH |
OAc |
863 |
T. baccata |
Apendino et al. (1994a) |
| |
|
|
|
|
|
|
|
|
|
|
T. media |
Rao et al. (1996b); |
| |
|
|
|
|
|
|
|
|
|
|
|
Hall et al. (1997) |
| 2α,7β,13α-Triacetoxy-5α,9α-dihydroxy-2(3→20)abeotaxa- |
C26H36O9 |
492.2359 |
A |
OAc |
OH |
OAc |
OH |
=O |
OAc |
4317 |
T. yunnanensis |
Shi et al. (2000b ) |
| 4(20),11-dien-10-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,7β-Diacetoxy-5α,10β,13α-trihydroxy-2(3→20)abeotaxa- |
C24H34O8 |
450.2254 |
A |
OAc |
OH |
OAc |
=O |
OH |
OH |
866 |
T. mairei |
Shi et al. (1999k) |
| 4(20),11-dien-9-one |
|
|
|
|
|
|
|
|
|
|
|
|
| Taxuspine W |
C26H36O9 |
492.2359 |
A |
OAc |
OH |
OAc |
=O |
OH |
OAc |
864 |
T. media |
Rao et al. (1996b); |
| |
|
|
|
|
|
|
|
|
|
|
T. cuspidata |
Hosoyama et al. (1996) |
| 2α,7β,10β-Triacetoxy-5α,13α-dihydroxy-2(3→20)abeotaxa- |
C26H36O9 |
492.2359 |
A |
OAc |
OH |
OAc |
=O |
OAc |
OH |
838 |
T. cuspidata |
Shi et al. (2004c) |
| 4(20),11-dien-9-one |
|
|
|
|
|
|
|
|
|
|
|
|
| Taxin B (5α-hydroxy-2α,7β,10β,13α-tetraacetoxy- |
C28H38O10 |
534.2465 |
A |
OAc |
OH |
OAc |
=O |
OAc |
OAc |
990 |
T. yunnanensis |
Yue et al. (1995) |
| 2(3→20)abeotaxa-4(20),11-dien-9-one) |
|
|
|
|
|
|
|
|
|
|
T. yunnanensis |
Shi et al. (1999f) |
| 2α,7β,10α-Triacetoxy-5α-hydroxy-2(3→20)abeotaxa- |
C26H34O9 |
490.2203 |
A |
OAc |
OH |
OAc |
=O |
OAc |
=O |
4326 |
T. sumatrana |
Luh et al. (2009) |
| 4(20),11-dien-9,13-dione |
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,5α,13α-Triacetoxy-7β,10β-dihydroxy-2(3→20)abeotaxa- |
C26H36O9 |
492.2359 |
A |
OAc |
OAc |
OH |
=O |
OH |
OAc |
989 |
T. mairei |
Shi and Oritani (2000) |
| 4(20),11-dien-9-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,5α,13α-Triacetoxy-7β-hydroxy-2(3→20)abeotaxa- |
C26H34O9 |
490.2203 |
A |
OAc |
OAc |
OH |
=O |
=O |
OAc |
987 |
T. mairei |
Oritani and Sugiyama |
| 4(20),11-diene-9,10-dione |
|
|
|
|
|
|
|
|
|
|
|
(1999) |
| 2α,5α,7β,13α-Tetraacetoxy-10β-hydroxy-2(3→20)abeotaxa- |
C28H38O10 |
534.2465 |
A |
OAc |
OAc |
OAc |
=O |
OH |
OAc |
988 |
T. mairei |
Shi et al. (1999k) |
| 4(20),11-dien-9-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 7-Deacetoxy-5-O-cinnamoyltaxin B (5α-cinnamoyloxy- |
C35H42O9 |
606.2829 |
A |
OAc |
a |
H |
=O |
OAc |
OAc |
837 |
T. canadensis |
Shi et al. (2003d) |
| 2α,10β,13α-triacetoxy-2(3→20)abeotaxa-3(4),11-dien-9-one) |
|
|
|
|
|
|
|
|
|
|
|
|
| Taxuspinanane H (deaminoacylcinnamoyltaxine A) |
C33H40O9 |
580.2672 |
A |
OAc |
a |
OH |
=O |
OH |
OAc |
865 |
T. cuspidata |
Morita et al. (1998a) |
| Taxuspine B |
C35H42O10 |
622.2778 |
A |
OAc |
a |
OAc |
=O |
OH |
OAc |
4304 |
T. cuspidata |
Kobayashi et al. (1994) |
| 5α-O-Cinnamoyltaxin B (5α-cinnamoyloxy-2α,7β,10β,13α-tetra- |
C37H44O11 |
664.2884 |
A |
OAc |
a |
OAc |
=O |
OAc |
OAc |
858 |
T. canadensis |
Zamir et al. (1999b) |
| acetoxy-2(3→20)abeotaxa-4(20),11-dien-9-one) |
|
|
|
|
|
|
|
|
|
|
T. yunnanensis |
Shi et al. (1999f) |
| Dantaxusin A |
C35H40O10 |
620.2621 |
A |
OAc |
a |
OAc |
=O |
=O |
OAc |
13914 |
T. yunnanensis |
Shinozaki et al. (2001) |
| 2α,7β,13α-Triacetoxy-5α-[(2'R,3'S)-N,N-dimethyl-3'-phenyl- |
C37H49NO10 |
667.3356 |
A |
OAc |
b |
OAc |
=O |
OH |
OAc |
862 |
T. cuspidata |
Shi et al. (1999g) |
| propanoyloxy]-10β-hydroxy-2(3→20)abeotaxa-4(20),11-dien- |
|
|
|
|
|
|
|
|
|
|
|
|
| 9-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,7β,13α-Triacetoxy-5α-[(2'R,3'S)-N,N-dimethyl- 3'-phenyl- |
C35H47NO10 |
641.3200 |
A |
OAc |
b |
OAc |
=O |
=O |
OAc |
903 |
T. cuspidata |
Shi et al. (2000c) |
| propanoyloxy]-2(3→20)abeotaxa-4(20),11-diene-9,10-dione |
|
|
|
|
|
|
|
|
|
|
|
|
| Taxine A |
C35H47NO10 |
641.3200 |
A |
OAc |
c |
OH |
=O |
OH |
OAc |
4307 |
T. baccata |
Graf and Berthold (1957) |
| |
|
|
|
|
|
|
|
|
|
|
|
Graf et al. (1982) |
| 7-O-Acetyltaxine A |
C37H49NO11 |
683.3306 |
A |
OAc |
c |
OAc |
=O |
OH |
OAc |
4311 |
T. baccata |
Barboni et al. (1995a) |
| |
|
|
|
|
|
|
|
|
|
|
T. yunnanensis |
Shi et al. (1999f) |
| 2α,7β,10β,13α-Tetraacetoxy-5α-[(2'R,3'S)-N,N-dimethyl- |
C39H51NO12 |
725.3411 |
A |
OAc |
c |
OAc |
=O |
OAc |
OAc |
859 |
T. yunnanensis |
Shi et al. (1999f) |
| 3'-phenylisoseryloxy]-2(3→20)abeotaxa-4(20),11-dien-9-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,7β-Diacetoxy-5α,10β,13β-trihydroxy-2(3→20)abeotaxa- |
C24H34O8 |
450.2254 |
B |
OAc |
OH |
OAc |
=O |
OH |
OH |
870 |
T. cuspidata |
Huo et al. (2007a) |
| 4(20),11-dien-9-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,10β,13α-Triacetoxy-2(3→20)abeotaxa-4(20),5,11-triene- |
C26H32O8 |
472.2097 |
C |
OAc |
n.a. |
=O |
=O |
OAc |
OAc |
4339 |
T. mairei |
Shi and Oritani (2000) |
| 7,9-dione |
|
|
|
|
|
|
|
|
|
|
|
|
| 2α,13α-Diacetoxy-10β-hydroxy-2(3→20)abeotaxa- |
C24H30O7 |
430.1992 |
C |
OAc |
n.a. |
=O |
=O |
OH |
OAc |
4335 |
T. mairei |
Shi and Oritani (2000) |
| 4(20),5,11-triene-7,9-dione |
|
|
|
|
|
|
|
|
|
|
|
|
| Taxumairone A |
C26H32O8 |
472.2097 |
D |
OAc |
=O |
n.a. |
=O |
OAc |
OAc |
869 |
T. mairei |
Shen et al. (2000c) |
| 2α,13α-Diacetoxy-10β-hydroxy-2(3→20)abeotaxa- |
C24H30O7 |
430.1992 |
D |
OAc |
=O |
n.a. |
=O |
OH |
OAc |
868 |
T. mairei |
Shi et al. (2006c) |
| 4(20),6,11-triene-5,9-dione |
|
|
|
|
|
|
|
|
|
|
|
|
| __________________________________________________________________________________________________________________________________________________________________ |
Table 21. Group V. 3,11-Cyclotaxanes.
____________________________________________________________________________________________________
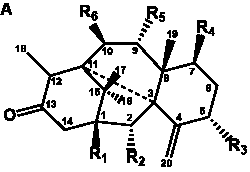
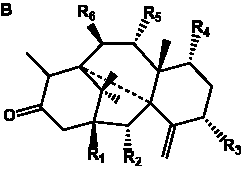
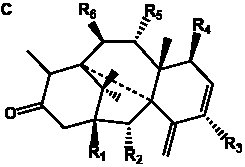
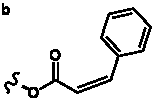
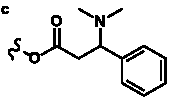
| Abbreviations: |
| OAc, O-acetyl |
| ______________________________________________________________________________________________________________________________________________________________ |
| |
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-1 |
C-2 |
C-5 |
C-7 |
C-9 |
C-10 |
Record |
Source(s) |
|
| ______________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 9α,10β-Diacetoxy-5α-cinnamoyloxy-3,11-cyclotax- |
C33H40O7 |
548.2774 |
A |
H |
H |
a |
H |
OAc |
OAc |
823 |
T. canadensis |
Shi et al. (2003b) |
| 4(20)-en-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 10β-Acetoxy-2α,5α,9α-trihydroxy-3,11-cyclotax-4(20)-en-13-one |
C22H32O6 |
392.2199 |
A |
H |
OH |
OH |
H |
OH |
OAc |
919 |
T. yunnanensis |
Kiyota et al. (2001) |
| 5-Cinnamoylphototaxicin II |
C29H36O6 |
480.2512 |
A |
H |
OH |
a |
H |
OH |
OH |
848 |
T. baccata |
Soto and Castedo (1998) |
| 10β-Acetoxy-5α-cinnamoyloxy-2α,9α-dihydroxy-3,11-cyclotax- |
C31H38O7 |
522.2618 |
A |
H |
OH |
a |
H |
OH |
OAc |
831 |
T. canadensis |
Shi et al. (2003d) |
| 4(20)-en-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 9α,10β-Diacetoxy-5α-cinnamoyloxy-2α-hydroxy-3,11-cyclotax- |
C33H42O8 |
566.2880 |
A |
H |
OH |
a |
H |
OAc |
OAc |
832 |
T. canadensis |
Shi et al. (2003d) |
| 4(20)-en-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 2α-Acetoxy-5α,9α,10β-trihydroxy-3,11-cyclotax-4(20)-en-13-one |
C22H32O6 |
392.2199 |
A |
H |
OAc |
OH |
H |
OH |
OH |
845 |
T. yunnanensis |
Shi et al. (2000b) |
| Taxinine K |
C26H36O8 |
476.2410 |
A |
H |
OAc |
OH |
H |
OAc |
OAc |
881 |
T. cuspidata |
Chiang et al. (1967) |
| Taxinine L |
C28H38O9 |
518.2516 |
A |
H |
OAc |
OAc |
H |
OAc |
OAc |
882 |
T. cuspidata |
Chiang et al. (1967) |
| 2α-Acetoxy-5α-cinnamoyloxy-9α,10β-dihydroxy-3,11-cyclotax- |
C31H38O7 |
522.2618 |
A |
H |
OAc |
a |
H |
OH |
OH |
839 |
T. canadensis |
Shi et al. (2004c) |
| 4(20)-en-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 2,10-Diacetyl-5-cinnamoylphototaxicin II (2α,10β-diacetoxy- |
C33H40O8 |
564.2723 |
A |
H |
OAc |
a |
H |
OH |
OAc |
920 |
T. canadensis |
Zamir et al. (1998) |
| 5α-cinnamoyloxy-9α-hydroxy-3,11-cyclotax-4(20)-en-13-one) |
|
|
|
|
|
|
|
|
|
|
T. yunnanensis |
Kiyota et al. (2001) |
| 2,9-Diacetyl-5-cinnamoylphototaxicin II |
C33H40O8 |
564.2723 |
A |
H |
OAc |
a |
H |
OAc |
OH |
849 |
T. canadensis |
Zamir et al. (1998) |
| Taxuspine C |
C35H42O9 |
606.2829 |
A |
H |
OAc |
a |
H |
OAc |
OAc |
981 |
T. cuspidata |
Kobayashi et al. (1994) |
| 2,10-Diacetyl-5-cinnamoyl-7β-hydroxyphototaxicin II |
C33H40O9 |
580.2672 |
A |
H |
OAc |
a |
OH |
OH |
OAc |
923 |
T. canadensis |
Zamir et al. (1998) |
| 3,11-cyclotax-4(20)-en-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 7β-Acetoxytaxuspine C |
C37H44O11 |
664.2884 |
A |
H |
OAc |
a |
OAc |
OAc |
OAc |
940 |
T. canadensis |
Zamir et al. (1999b) |
| 10β-Acetoxy-5α-cinnamoyloxy-1β,2α,9α-trihydroxy- |
C31H38O8 |
538.2567 |
A |
OH |
OH |
a |
H |
OH |
OAc |
840 |
T. canadensis |
Petzke et al. (2004) |
| 3,11-cyclotax-4(20)-en-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 5-Cinnamoyl-10-acetylphototaxicin I |
C31H38O8 |
538.2567 |
A |
OH |
OH |
a |
H |
OH |
OAc |
4315 |
T. baccata |
Appendino et al. (1992c) |
| 5-Cinnamoyl-9-acetylphototaxicin I |
C31H38O8 |
538.2567 |
A |
OH |
OH |
a |
H |
OAc |
OH |
896 |
T. baccata |
Appendino et al. (1994c) |
| 2α,10β-Diacetoxy-5α-cinnamoyloxy-1β,9α-dihydroxy- |
C33H42O9 |
582.2829 |
A |
OH |
OAc |
a |
H |
OH |
OAc |
821 |
T. baccata |
Shi et al. (2004a) |
| 3,11-cyclotax-4(20)-en-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 1β-Hydroxytaxuspine C |
C35H42O10 |
622.2778 |
A |
OH |
OAc |
a |
H |
OAc |
OAc |
928 |
T. cuspidata |
Shi et al. (1999e) |
| 2,10-Diacetyl-5-[(Z)-cinnamoyl]phototaxicin II |
C33H40O8 |
564.2723 |
A |
H |
OAc |
b |
H |
OH |
OAc |
924 |
T. canadensis |
Yang et al. (2009) |
| 2α,10β-Diacetoxy-9α-hydroxy-5α-(3'-dimethylamino-3'- |
C35H47NO8 |
609.3302 |
A |
H |
OAc |
c |
H |
OH |
OAc |
925 |
T. canadensis |
Shi et al. (2006d) |
| phenylpropanoyloxy)-3,11-cyclotax-4(20)-en-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
| Taxuspine H |
C37H49NO9 |
651.3407 |
A |
H |
OAc |
c |
H |
OAc |
OAc |
936 |
T. cuspidata |
Kobayashi et al. (1995a) |
| 2α,7α,10β-Triacetoxy-5α-cinnamoyloxy-9α-hydroxy- |
C35H42O10 |
622.2778 |
B |
H |
OAc |
a |
OAc |
OH |
OAc |
822 |
T. canadensis |
Shi et al. (2003b) |
| 3,11-cyclotax-4(20)-en-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 7β,10β-Diacetoxy-9α-hydroxy-3,11-cyclotax- |
C24H32O6 |
416.2199 |
C |
H |
H |
H |
OAc |
OH |
OAc |
3929 |
T. cuspidata |
Wang et al. (2013) |
| 4(20),5-dien-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
| 3α,11α-cyclotaxinine NN-1 |
C26H34O7 |
458.2305 |
C |
H |
OAc |
H |
H |
OAc |
OAc |
956 |
T. cuspidata |
Wang et al. (2008) |
| 2α,7β,10β-Triacetoxy-5-cinnamyloxy-9α-hydroxy-3α,11α- |
C35H40O10 |
620.2622 |
C |
H |
OAc |
a |
OAc |
OH |
OAc |
3930 |
T. cuspidata |
Wang et al. (2013) |
| cyclotaxa-4(20),5-dien-13-one |
|
|
|
|
|
|
|
|
|
|
|
|
| ______________________________________________________________________________________________________________________________________________________________ |
Table 22. Groups VI - IX. Tetra-, penta- and hexacyclic taxanes.
____________________________________________________________________________________________________
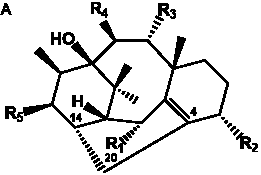
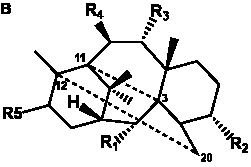
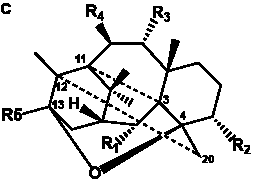
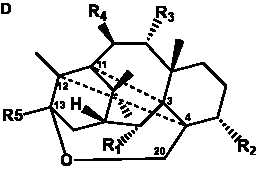
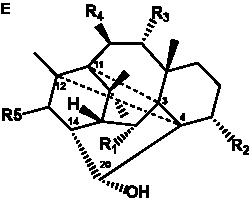
| Abbreviations: |
| OAc, O-acetyl |
| ____________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-2 |
C-5 |
C-9 |
C-10 |
C-13 |
Record |
Source(s) |
|
| ____________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
| Group VI. 14,20-Cyclotaxanes |
|
|
|
|
|
|
|
|
|
|
|
| 2α,9α-Diacetoxy-5α-cinnamoyloxy-10β,11β-dihydroxy-14β,20-cyclotax- |
C33H40O9 |
580.2672 |
A |
OAc |
a |
OAc |
OH |
=O |
833 |
T. canadensis |
Shi et al. (2002) |
| 3-en-13-one |
|
|
|
|
|
|
|
|
|
|
|
| Group VII. 3,11:12,20-Dicyclotaxanes |
|
|
|
|
|
|
|
|
|
|
|
| 2α,9α,10β-Triacetoxy-5α-hydroxy-3,11-cyclo-12,20-cyclotaxa-13-one |
C26H36O8 |
476.2410 |
B |
OAc |
OH |
OAc |
OAc |
=O |
779 |
T. canadensis |
Shi et al. (2003e) |
| (canataxapropellane; note: this is not canataxpropellane) |
|
|
|
|
|
|
|
|
|
|
|
| Dipropellane B |
C24H34O8 |
450.2254 |
C |
OH |
OH |
OAc |
OAc |
OH |
774 |
T. canadensis |
Shi et al. (2004d) |
| Dipropellane A |
C24H34O8 |
450.2254 |
C |
OAc |
OH |
OH |
OAc |
OH |
918 |
T. canadensis |
Shi et al. (2004d) |
| Dipropellane C |
C26H36O9 |
492.2359 |
C |
OAc |
OH |
OAc |
OAc |
OH |
778 |
T. canadensis |
Shi et al. (2004d) |
| Group VIII. 3,11:4,12-Dicyclotaxanes |
|
|
|
|
|
|
|
|
|
|
|
| Taxpropellane A |
C24H35O8 |
451.2332 |
D |
OAc |
OH |
OH |
OAc |
OH |
4085 |
T. canadensis |
Zhang et al. (2008d) |
| Group IX. 3,11:4,12:14,20_Tricyclotaxanes |
|
|
|
|
|
|
|
|
|
|
|
| Canataxpropellane (2α,10β-diacetoxy-5α,9α,20α-trihydroxy- |
C24H32O8 |
448.2097 |
E |
OAc |
OH |
OH |
OAc |
=O |
4054 |
T. canadensis |
Huo et al. (2007d) |
| 3α,11α;4α,12α;14α,20-tricyclotaxan-13-one |
|
|
|
|
|
|
|
|
|
|
|
| ________________________________________________________________________________________________________________________________ |
Table 23. Group X. Bicyclic taxanes. 3,8-Secotaxa-3,8,11-trienes.
____________________________________________________________________________________________________
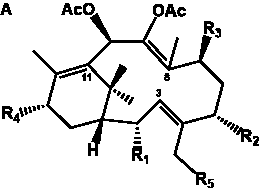
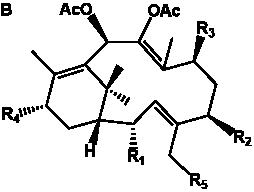
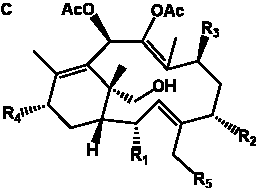
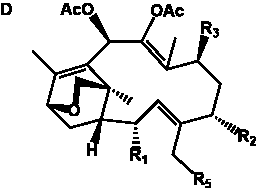
| Abbreviations: |
| OAc, O-acetyl |
| OBz, O-benzoyl |
| =O, ketone functional group |
| n.a., not applicable |
| _______________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-2 |
C-5 |
C-7 |
C-13 |
C-20 |
Record |
Source(s) |
|
| _______________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
| Tasumatrol M |
C28H40O11 |
552.2571 |
A |
OH |
OH |
OH |
OAc |
OAc |
13081 |
T. sumatrana |
Shen et al. (2005b) |
| 5-Deacetyltaxachitriene B |
C28H40O11 |
552.2571 |
A |
OH |
OH |
OAc |
OAc |
OH |
897 |
T. chinensis |
Fang et al. (1996) |
| 2-Deacetyltaxachitriene A |
C30H42O12 |
594.2676 |
A |
OH |
OH |
OAc |
OAc |
OAc |
809 |
T. chinensis |
Shi et al. (1998e) |
| (3E,8E)-7β,9,10β-Triacetoxy-2α,5α,20-trihydroxy- |
C26H36O10 |
508.2308 |
A |
OH |
OH |
OAc |
=O |
OH |
995 |
T. mairei |
Shi et al. (1999l) |
| 3,8-secotaxa-3,8,11-trien-13-one |
|
|
|
|
|
|
|
|
|
|
|
| Taxuspine U |
C28H40O11 |
552.2571 |
A |
OH |
OAc |
OH |
OAc |
OH |
807 |
T. cuspidata |
Hosoyama et al. (1996) |
| Taxachitriene B |
C30H42O13 |
610.2625 |
A |
OH |
OAc |
OAc |
OAc |
OH |
811 |
T. chinensis |
Fang et al. (1995) |
| 2,7-Dideacetyltaxuspine X |
C37H46O12 |
682.2989 |
A |
OH |
a |
OH |
OAc |
OAc |
993 |
T. cuspidata |
Shi et al. (1999m) |
| |
|
|
|
|
|
|
|
|
|
T. mairei |
Li et al. (2006) |
| (3E,8E)-9,10β,13α-Triacetoxy-2α,7β,20-trihydroxy-5α- |
C35H44O11 |
640.2884 |
A |
OH |
a |
OH |
OAc |
OH |
802 |
T. sumatrana |
Luh et al. (2009) |
| [(E)-cinnamoyloxy]-3,8-secotaxa-3,8,11-triene |
|
|
|
|
|
|
|
|
|
|
|
| 2,20-Dideacetyltaxuspine X |
C37H46O12 |
682.2989 |
A |
OH |
a |
OAc |
OAc |
OH |
991 |
T. cuspidata |
Shi et al. (1999m) |
| 2-Deacetyltaxuspine X |
C39H48O13 |
724.3095 |
A |
OH |
a |
OAc |
OAc |
OAc |
992 |
T. cuspidata |
Shi et al. (1999m) |
| Tasumatrol N |
C28H38O11 |
550.2414 |
A |
OH |
=O |
OH |
OAc |
OAc |
13082 |
T. sumatrana |
Shen et al. (2005b) |
| 7-Deacetylcanadensene |
C28H40O11 |
552.2571 |
A |
OAc |
OH |
OH |
OAc |
OH |
898 |
T. mairei |
Shi et al. (1999o) |
| |
|
|
|
|
|
|
|
|
|
T. sumatrana |
Shen et al. (2005b) |
| 7-Deacetyltaxachitrine A ((3E,8E)-2α,9,10β,13α,20-penta- |
C30H42O12 |
594.2676 |
A |
OAc |
OH |
OH |
OAc |
OAc |
806 |
T. mairei |
Shi et al. (1999p) |
| acetoxy-5α,7β-dihydroxy-3,8-secotaxa-3,8,11-triene) |
|
|
|
|
|
|
|
|
|
T. sumatrana |
Luh et al. (2009) |
| 13-Deacetylcanadensene |
C28H40O11 |
552.2571 |
A |
OAc |
OH |
OAc |
OH |
OH |
13958 |
T. mairei |
Shi et al. (1999m) |
| 13-Deacetyltaxachitrine A |
C30H42O12 |
594.2676 |
A |
OAc |
OH |
OAc |
OH |
OAc |
997 |
T. mairei |
Shi et al. (1999p) |
| 5-epi-Canadensene |
C30H42O12 |
594.2676 |
A |
OAc |
OH |
OAc |
OAc |
OH |
808 |
T. canadensis |
Zamir et al. (1998) |
| |
|
|
|
|
|
|
|
|
|
T. mairei |
Li et al. (2006) |
| Taxachitriene A |
C32H44O13 |
636.2782 |
A |
OAc |
OH |
OAc |
OAc |
OAc |
810 |
T. chinensis |
Fang et al. (1995) |
| 20-O-Cinnamoyl-5-epi-canadensene |
C39H48O13 |
724.3095 |
A |
OAc |
OH |
OAc |
OAc |
a |
800 |
T. canadensis |
Shi et al. (2003f) |
| (3E,8E)-2α,7β-9,10β,20-Pentaacetoxy-5α-hydroxy- |
C30H40O12 |
592.2520 |
A |
OAc |
OH |
OAc |
=O |
OAc |
994 |
T. mairei |
Shi et al. (1999l) |
| 3,8-secotaxa-3,8,11-trien-13-one |
|
|
|
|
|
|
|
|
|
|
|
| (3E,8E)-2α,5α,7β,9,10β,13α-Hexaacetoxy-20-hydroxy- |
C32H44O13 |
636.2782 |
A |
OAc |
OAc |
OAc |
OAc |
OH |
803 |
T. sumatrana |
Luh et al. (2009) |
| 3,8-secotaxa-3,8,11-triene |
|
|
|
|
|
|
|
|
|
|
|
| (3E,8E)-2α,9,10β,13α,20-Penta-acetoxy-7β-hydroxy- |
C30H40O12 |
592.2520 |
A |
OAc |
=O |
OH |
OAc |
OAc |
804 |
T. sumatrana |
Luh et al. (2009) |
| 3,8-secotaxa-3,8,11-trien-13-one |
|
|
|
|
|
|
|
|
|
|
|
| 5-epi-O-Cinnamoylcanadensene |
C39H48O13 |
724.3095 |
A |
OAc |
a |
OAc |
OAc |
OH |
813 |
T. canadensis |
Zhang et al. (2000a) |
| Taxuspine X |
C41H50O14 |
766.3201 |
A |
OAc |
a |
OAc |
OAc |
OAc |
812 |
T. cuspidata |
Shigemori et al. (1997) |
| (3E,8E)-2α,7β,9,10β,13α,20-Hexaacetoxy-5-[2'-acetoxy- |
C43H52O16 |
824.3255 |
A |
OAc |
b |
OAc |
OAc |
OAc |
801 |
T. mairei |
Shi et al. (1999n) |
| cinnamoyloxy]-3,8-secotaxa-3,8,11-triene |
|
|
|
|
|
|
|
|
|
|
|
| 5-(N,N-Dimethyl-3′-phenylisoseryl)taxachitriene A |
C47H57NO15 |
875.3728 |
A |
OAc |
c |
OAc |
OAc |
OAc |
984 |
T. mairei |
Shi et al. (1998b) |
| Canadensene (20-deacetyltaxachitriene A) |
C30H42O12 |
594.2676 |
B |
OAc |
OH |
OAc |
OAc |
OH |
815 |
T. canadensis |
Zamir et al. (1995b) |
| Taxumairol M |
C32H44O14 |
652.2731 |
C |
OAc |
OAc |
OH |
OAc |
OAc |
998 |
T. mairei |
Shen et al. (1999b) |
| Canataxpyran A |
C28H38O11 |
550.2414 |
D |
OH |
OH |
OAc |
n.a. |
OAc |
3971 |
T. canadensis |
Li et al. (2012) |
| _______________________________________________________________________________________________________________________________________________________ |
Table 24. Groups X, XI. Bicyclic taxanes. 3,8-Secotaxa-3,7,11-trienes, 3,8-secotaxa-3,7,12-trienes, 11,12-secotaxanes.
____________________________________________________________________________________________________
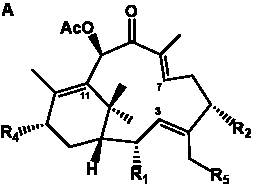
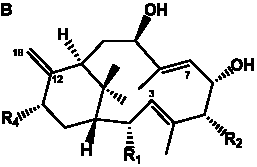
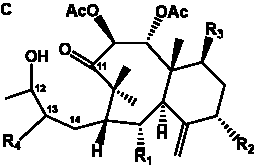
| Abbreviations: |
| OAc, O-acetyl |
| OBz, O-benzoyl |
| n.a., not applicable |
| _______________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
| Compound |
Molecular |
Monoisotopic |
Core |
R1 |
R2 |
R3 |
R4 |
R5 |
Spektraris |
Plant |
References |
| |
Formula |
Mass |
|
C-2 |
C-5 |
C-7 |
C-13 |
C-20 |
Record |
Source(s) |
|
| _______________________________________________________________________________________________________________________________________________________________ |
| |
|
|
|
|
|
|
|
|
|
|
|
| Group X. 3,8-Secotaxa-3,7,11-trienes, 3,8-secotaxa-3,7,12-trienes |
| (3E,7E)-10β,13α-Diacetoxy-2α,5α,20-trihydroxy- 3,8-secotaxa-3,7,11- |
C24H34O8 |
450.2254 |
A |
OH |
OH |
n.a. |
OAc |
OH |
4164 |
T. mairei |
Shi et al. (1999p) |
| trien-9-one |
|
|
|
|
|
|
|
|
|
|
|
| (3E,7E)-2α,10β-Diacetoxy-5α,13α,20-trihydroxy-3,8-secotaxa-3,7,11- |
C24H34O8 |
450.2254 |
A |
OAc |
OH |
n.a. |
OH |
OH |
4165 |
T. chinensis |
Shi et al. (1998e) |
| trien-9-one |
|
|
|
|
|
|
|
|
|
|
|
| (3E,7E)-2α,10β,13α-Triacetoxy-5α,20-dihydroxy-3,8- secotaxa-3,7,11- |
C26H36O9 |
492.2359 |
A |
OAc |
OH |
n.a. |
OAc |
OH |
4166 |
T. chinensis |
Shi et al. (1998e) |
| trien-9-one |
|
|
|
|
|
|
|
|
|
|
|
| (3E,7E)-2α,10β,13α,20-Tetraacetoxy-5α-hydroxy-3,8-secotaxa-3,7,11- |
C28H38O10 |
534.2465 |
A |
OAc |
OH |
n.a. |
OAc |
OAc |
4150 |
T. mairei |
Shi et al. (1999p) |
| trien-9-one |
|
|
|
|
|
|
|
|
|
|
|
| (3E,7E)-2α,5α,10αβ,13α-Tetraacetoxy-20-hydroxy-3,8- secotaxa-3,7,11- |
C28H38O10 |
534.2465 |
A |
OAc |
OAc |
n.a. |
OAc |
OH |
4159 |
T. mairei |
Shi et al. (1999p) |
| trien-9-one |
|
|
|
|
|
|
|
|
|
|
|
| (3E,7E)-2α,5α,10β,13α,20-Pentaacetoxy-3,8-secotaxa-3,7,11-trien-9-one |
C30H40O11 |
576.2571 |
A |
OAc |
OAc |
n.a. |
OAc |
OAc |
4169 |
T. mairei |
Shi et al. (1999q) |
| (11αH)-3,8-Secotaxa-3,7,12(18)-triene-2α,6α,9β-triol |
C20H32O3 |
320.2351 |
B |
OH |
H,H |
n.a. |
H,H |
H |
4209 |
T. mairei |
Shi et al. (2005b) |
| Group XI. 11,12-Secotaxanes |
|
|
|
|
|
|
|
|
|
|
|
| Taxusecone |
C28H40O12 |
568.2520 |
C |
OAc |
OH |
OAc |
=O |
n.a. |
4231 |
T. cuspidata |
Yu et al. (2009) |
| _______________________________________________________________________________________________________________________________________________________________ |
REFERENCES
Ando, M., Sakai, J.I., Zhang, S., Watanabe, Y., Kosugi, K., Suzuki, T., Hagiwara, H., 1997. A new basic taxoid from Taxus cuspidata. J. Nat. Prod. 60, 499-501. https://doi.org/10.1021/np9606963
Appendino, G., Gariboldi, P., Pisetta, A., Bombardelli, E., Gabetta, B., 1992a. Taxanes from Taxus baccata. Phytochemistry 31, 4253-4257. https://doi.org/10.1016/0031-9422(92)80454-M
Appendino, G., Gariboldi, P., Gabetta, B., Pace, R., Bombardelli, E., Viterbo, D., 1992b. 14β-Hydroxy-10-deacetylbaccatin III, a new taxane from Himalayan yew (Taxus wallichiana Zucc.). J. Chem. Soc. Perkin Transact. 1, 2925-2929. https://doi.org/10.1039/P19920002925
Appendino, G., Lusso, P., Gariboldi, P., Bombardelli, E., Gabetta, B., 1992c. A 3, 11-cyclotaxane from Taxus baccata. Phytochemistry 31, 4259-4262. https://doi.org/10.1016/0031-9422(92)80455-N
Appendino, G., Tagliapietra, S., Özen, H.Ç., Gariboldi, P., Gabetta, B., Bombardelli, E., 1993a. Taxanes from the seeds of Taxus baccata. J. Nat. Prod. 56, 514-520. https://doi.org/10.1021/np50094a010
Appendino, G., Barboni, L., Gariboldi, P., Bombardelli, E., Gabetta, B., Viterbo, D., 1993b. Revised structure of brevifoliol and some baccatin VI derivatives. J. Chem. Soc. Chem. Commun. 1587-1588. https://doi.org/10.1039/C39930001587
Appendino, G., Özen, H.C., Fenoglio, I., Gariboldi, P., Gabetta, B., Bombardelli, E., 1993c. Pseudoalkaloid taxanes from Taxus baccata. Phytochemistry 33, 1521-1523. https://doi.org/10.1016/0031-9422(93)85125-B
Appendino, G., Özen, H.Ç., Gariboldi, P., Gabetta, B., 1993d. Four new taxanes from the needles of Taxus baccata. Fititerapia-Milano 64 (S1), 47-51.
Appendino, G., Özen, H.Ç., Gariboldi, P., Torregiani, E., Gabetta, B., Nizzola, R., Bombardelli, E., 1993e. New oxetane-type taxanes from Taxus wallichiana Zucc. J. Chem. Soc. Perkin Transact. 1, 1563-1566. https://doi.org/10.1039/P19930001563
Appendino, G., Cravotto, G., Enriù, R., Jakupovic, J., Gariboldi, P., Gabetta, B., Bombardell, E., 1994a. Rearranged taxanes from Taxus baccata. Phytochemistry 36, 407-411. https://doi.org/10.1016/S0031-9422(00)97085-7
Appendino, G., Cravotto, G., Enriù, R., Gariboldi, P., Barboni, L., Torregiani, E., Gabetta, B., Zini, G., Bombardelli, E., 1994b. Taxoids from the roots of Taxus × media cv. Hicksii. J. Nat. Prod. 57, 607-613. https://doi.org/10.1021/np50107a007
Appendino, G., Ozen, H.C., Gariboldi, B., Gabetta, B., Bombardelli, E. 1994c. Title not available. Fitoterapia (Milano) 64 (Suppl. 1), 47-51.
Appendino, G., 1995. The phytochemistry of the yew tree. Nat. Prod. Rep. 12, 349-360. https://doi.org/10.1039/NP9951200349
Balza, F., Tachibana, S., Barrios, H., Towers, G.N., 1991. Brevifoliol, a taxane from Taxus brevifolia. Phytochemistry 30, 1613-1614. https://doi.org/10.1016/0031-9422(91)84218-H
Baloglu, E., and Kingston, D.G., 1999. The taxane diterpenoids. J. Nat. Prod. 62, 1448-1472. https://doi.org/10.1021/np990176i
Banskota, A.H., Usia, T., Tezuka, Y., Kouda, K., Nguyen, N.T., Kadota, S., 2002. Three new C-14 oxygenated taxanes from the wood of Taxus yunnanensis. J. Nat. Prod. 65, 1700-1702. https://doi.org/10.1021/np020235j
Barboni, L., Gariboldi, P., Torregiani, E., Appendino, G., Gabetta, B., Zini, G., Bombardelli, E., 1993. Taxanes from the needles of Taxus wallichiana. Phytochemistry 33, 145-150. https://doi.org/10.1016/0031-9422(93)85411-J
Barboni, L., Gariboldi, P., Torregiani, E., Appendino, G., Gabetta, B., Bombardelli, E., 1994a. Taxol analogues from the roots of Taxus x media. Phytochemistry 36, 987-990. https://doi.org/10.1016/S0031-9422(00)90476-X
Barboni, L., Gariboldi, P., Torregiani, E., Appendino, G., Cravotto, G., Bombardelli, E., Gabetta, B., Viterbo, D., 1994b. Chemistry and occurrence of taxane derivatives. Part 16. Rearranged taxoids from Taxus × media Rehd. cv Hicksii. X-Ray molecular structure of 9-O-benzoyl-9, 10-dide-O-acetyl-11 (15→1)abeo-baccatin VI. J. Chem. Soc. Perkin Transact. 1, 3233-3238. https://doi.org/10.1039/P19940003233
Barboni, L., Gariboldi, P., Appendino, G., Enriù, R., Gabetta, B., Bombardelli, E., 1995a. New taxoids from Taxus baccata L. Liebigs Ann. Chem. 1995, 345-349. https://doi.org/10.1002/jlac.199519950243
Barboni, L., Gariboldi, P., Torregiani, E., Appendino, G., Varese, M., Gabetta, B., Bombardelli, E., 1995b. Minor taxoids from Taxus wallichiana. J. Nat. Prod. 58, 934-939. https://doi.org/10.1021/np50120a019
Baxter, J., Lythgoe, B., Scales, B., Scrowston, R., Trippett, S., 1962. Taxine. Part I. Isolation studies and the functional groups of O-cinnamoyltaxicin-I. J. Chem. Soc. 2964-2971. https://doi.org/10.1039/JR9620002964
Beutler, J.A., Chmurny, G.M., Look, S.A., Witherup, K.M., 1991. Taxinine M, a new tetracyclic taxane from Taxus brevifolia. J. Nat. Prod. 54, 893-897. https://doi.org/10.1021/np50075a028
Bull, J.A., Croft, R.A., Davis, O.A., Doran, R. and Morgan, K.F., 2016. Oxetanes: recent advances in synthesis, reactivity, and medicinal chemistry. Chem. Rev. 116, 12150-12233. https://doi.org/10.1021/acs.chemrev.6b00274
Bundy, J.G., Davey, M.P. and Viant, M.R., 2009. Environmental metabolomics: a critical review and future perspectives. Metabolomics 5, 3-21. https://doi.org/10.1007/s11306-008-0152-0
Cao, C.M., Zhang, M. L., Wang, Y.F., Shi, Q.W., Yamada, T., Kiyota, H., 2006. Two new taxanes from the needles and branches bark of Taxus cuspidata. Chem. Biodiv. 3, 1153-1161. https://doi.org/10.1002/cbdv.200690117
Chan, W.R., Halsall, T.G., Hornby, G.M., Oxford, A.W., Sabel, W., Bjåmer, K., Ferguson, G., Robertson, J.M., 1966. Taxa-4(16),11-diene-5α,9α,10β,13α-tetraol, a new taxane derivative from the heartwood of yew (T. baccata L.): X-ray analysis of a p-bromobenzoate derivative. Chem. Commun. (London), 923-925. https://doi.org/10.1039/C19660000923
Chattopadhyay, S.K., Sharma, R.P., Appendino, G., Gariboldi, P., 1995. A rearranged taxane from the Himalayan yew. Phytochemistry 39, 869-870. https://doi.org/10.1016/0031-9422(95)00132-Q
Chattopadhyay, S.K., Saha, G.C., Sharma, R.P., Kumar, S., Roy, R., 1996. A rearranged taxane from the himalayan yew Taxus wallichiana. Phytochemistry 42, 787-788. https://doi.org/10.1016/0031-9422(95)00934-5
Chattopadhyay, S.K., Tripathi, V., Sharma, R.P., Shawl, A., Joshi, B.S., Roy, R., 1999. A brevifoliol analogue from the Himalyan yew Taxus wallichiana. Phytochemistry 50, 131-133. https://doi.org/10.1016/S0031-9422(98)00461-0
Chattopadhyay, S.K., Pal, A., Maulik, P.R., Kaur, T., Garg, A., Khanuja, S.P.S., 2006. Taxoid from the needles of the Himalayan yew Taxus wallichiana with cytotoxic and immunomodulatory activities. Bioorg. Med. Chem. Lett. 16, 2446-2449. https://doi.org/10.1016/j.bmcl.2006.01.077
Chau, M. and Croteau, R., 2004. Molecular cloning and characterization of a cytochrome P450 taxoid 2α-hydroxylase involved in Taxol biosynthesis. Arch. Biochem. Biophys. 427, 48-57. https://doi.org/10.1016/j.abb.2004.04.016
Chau, M.D., Walker, K., Long, R., Croteau, R., 2004a. Regioselectivity of taxoid-O-acetyltransferases: heterologous expression and characterization of a new taxadien-5α-ol-O-acetyltransferase. Arch. Biochem. Biophys. 430, 237-246. https://doi.org/10.1016/j.abb.2004.07.013
Chau, M.D., Jennewein, S., Walker, K., Croteau, R., 2004b. Taxol biosynthesis: molecular cloning and characterization of a cytochrome P450 taxoid 7β-hydroxylase. Chem. Biol. 11, 663-672. https://doi.org/10.1016/j.chembiol.2004.02.025
Chauvière, G., Guènard, D., Picot, F., Senilh, V., Potier, P., 1981. Analyse structurale et étude biochimique de produits isolés de l'if: Taxus baccata L. (Taxacées). C. R. Acad. Sci. 293, 501-503.
Chauvière, G., Guènard, D., Pascard, C., Picot, F., Potier, P., Prangé, T., 1982. Taxagifine: new taxane derivative from Taxus baccata L.(Taxaceae). J. Chem. Soc. Chem Commun. 9, 495-496. https://doi.org/10.1039/C39820000495
Chaw, S.M., Long, H., Wang, B.S., Zharkikh, A. and Lie, W.H., 1993. The phylogenetic position of Taxaceae based on 18S rRNA sequences. J. Mol. Evol. 37, 624-630. https://doi.org/10.1007/BF00182748
Chen, R., and Kingston, D.G., 1994. Isolation and structure elucidation of new taxoids from Taxus brevifolia. J. Nat. Prod. 57, 1017-1021. https://doi.org/10.1021/np50109a025
Chen, W.M., Chang, P. L., Wu, B., Zheng, Q.T., 1991. Studies on the chemical constituents of Taxus yunnanensis. Chin. Chem. Lett. 2, 441-442.
Chen, W.M., Zhou, J.Y., Zhang, P.L., Fang, Q.C., 1993a. Yunnanxol and yunnanxamine-two new tetracyclic taxane diterpenoids from Taxus yunnanensis. Chin. Chem. Lett. 4, 699.
Chen, W.M., Zou, J.Y., Zhang, P.L., Fang, Q.C., 1993b. Taxayuntin A, B, C and D-four new tetracyclic taxanes from Taxus yunnanensis. Chin. Chem. Lett. 4, 695-695.
Chen, W., Zhang, P., Zhou, J., 1994a. Isolation and structural elucidation of four new taxane diterpenoids from Taxus yunnanensis. Acta Pharm. Sin. 3.
Chen, W., Zhang, P., Zhou, J., Liu, X., Fang, Q., 1994b. Chemical studies of Taxus yunnanensis IV: Isolation and structure elucidation of two new taxane diterpenoids. Acta Pharm. Sin. 29, 757.
Chen, Y.J., Chen, C.Y., Shen, Y.C., 1999. New taxoids from the seeds of Taxus chinensis. J. Nat. Prod. 62, 149-151. https://doi.org/10.1021/np9802365
Chen, K., Liu, X.Q., Wang, W.L., Luo, J.G., Kong, L.Y., 2020. Taxumarienes A–G, seven new α-glucosidase inhibitory taxane-diterpenoids from the leaves of Taxus mairei. Bioorg. Chem. 94, 103400. https://doi.org/10.1016/j.bioorg.2019.103400
Cheng, K., Fang, W., Yang, Y., Xu, H., Meng, C., Kong, M., He, W., Fang, Q., 1996. C-14 Oxygenated taxanes from Taxus yunnanensis cell cultures. Phytochemistry 42, 73-75. https://doi.org/10.1016/0031-9422(95)00840-3
Cheng, Q., Oritani, T., Horiguchi, T., 2000. Two novel taxane diterpenoids from the needles of Japanese yew, Taxus cuspidata. Biosci. Biotechnol. Biochem. 64, 894-898. https://doi.org/10.1271/bbb.64.894
Chiang, H., Woods, M., Nakadaira, Y., Nakanishi, K., 1967. The structures of four new taxinine congeners, and a photochemical transannular reaction. Chem. Commun. (London), 1201-1202. https://doi.org/10.1039/C19670001201
Chiang, H., 1975. The constituents of Taxus chinensis Rehd. Shi Ta Hsueh Pao 20, 147.
Choudhary, M.I., Khan, A.M., Ashraf, M., 2002. Two New Rearranged Taxoids from Taxus wallichiana Zucc. Chem. Pharm. Bull. 50, 1488-1490. https://doi.org/10.1248/cpb.50.1488
Chu, A., Zajicek, J., Davin, L.B., Lewis, N.G., Croteau, R.B., 1992. Mixed acetoxy-benzoxy taxane esters from Taxus brevifolia. Phytochemistry. 31, 4249-4252. https://doi.org/10.1016/0031-9422(92)80453-L
Chu, A., Zajicek, J., Towers, G.N., Soucy-Breau, C.M., Lewis, N.G., Croteau, R., 1993a. Brevifoliol: a structure revision. Phytochemistry 34, 269-271. https://doi.org/10.1016/S0031-9422(00)90817-3
Chu, A., Davin, L.B., Zajicek, J., Lewis, N.G., Croteau, R., 1993b. Intramolecular acyl migrations in taxanes from Taxus brevifolia. Phytochemistry. 34, 473-476. https://doi.org/10.1016/0031-9422(93)80033-O
Chu, A., Furlan, M., Davin, L.B., Zajicek, J., Towers, G.N., Soucy-Breau, C.M., Rettig, S.J., Croteau, R., Lewis, N.G., 1994. Phenylbutanoid and taxane-like metabolites from needles of Taxus brevifolia. Phytochemistry 36, 975-985. https://doi.org/10.1016/S0031-9422(00)90475-8
Collins, D., Mill, R.R., Möller, M., 2003. Species separation of Taxus baccata, T. canadensis, and T. cuspidata (Taxaceae) and origins of their reputed hybrids inferred from RAPD and cpDNA data. Am. J. Bot. 90, 175-182. https://doi.org/10.3732/ajb.90.2.175
Cope, E.A., 1998. Taxaceae: the genera and cultivated species. Bot. Rev. 64, 291-322.
Coughlan, P., Carolan, J.C., Hook, I.L., Kilmartin, L., Hodkinson, T.R., 2020. Phylogenetics of Taxus using the internal transcribed spacers of nuclear ribosomal DNA and plastid trnL-F regions. Horticulturae 6, 19. https://doi.org/10.3390/horticulturae6010019
Dang, P.H., Nguyen, H.H., Truong, H.T., Do, T.N., Nguyen, H.X., Nguyen, M.T., Abe, M., Takagi, R., Nguyen, N.T., 2017. Two ring opened oxetane taxoids containing a C-20 benzoyloxy group from the roots of Taxus wallichiana Zucc. Tetrahedron Lett. 58, 3897-3900. https://doi.org/10.1016/j.tetlet.2017.08.073
Das, B., Rao, S.P., Srinivas, K., Yadav, J., Das, R., 1995. A taxoid from needles of Himalayan Taxus baccata. Phytochemistry 38, 671-674. https://doi.org/10.1016/0031-9422(94)00751-E
De Bellis, P., Lovati, M., Pace, R., Peterlongo, F., Zini, G., 1995. Isolation of 7-epi-cephalomannine from Taxus x media cv.'Hicksii' needles. Fitoterapia (Milano) 66, 521-524.
Della Casa de Marcano, D., and Halsall, T., 1969. The isolation of seven new taxane derivatives from the heartwood of yew (Taxus baccata L.). J. Chem. Soc. Chem. Commun. 1282-1283. https://doi.org/10.1039/C29690001282
Della Casa de Marcano, D., and Halsall, T., 1970. The structure of the diterpenoid baccatin-I, the 4β,20-epoxide of 2α,5α,7β,9α,10β,13α-hexa-acetoxytaxa-4(20),11-diene. J. Chem. Soc. Chem. Commun 21, 1381-1382. https://doi.org/10.1039/C29700001381
Della Casa de Marcano, D., Halsall, T., Hornby, G., 1970a. The structure of baccatin-III, a partially esterified octahydroxy-monoketo-taxane derivative lacking a double bond at C-4. J. Chem. Soc. Chem. Commun. 216-217. https://doi.org/10.1039/C2970000216B
Della Casa de Marcano, D., Halsall, T., Castellano, E., and Hodder, O., 1970b. Crystallographic structure determination of the diterpenoid baccatin-V, a naturally occurring oxetan with a taxane skeleton. J. Chem. Soc. Chem. Commun. 1382-1383. https://doi.org/10.1039/C29700001382
Della Casa De Marcano, D.P., and Halsall, T.G., 1975. Structures of some taxane diterpenoids, baccatins-III,-IV,-VI, and-VII and 1-dehydroxybaccatin-IV, possessing an oxetan ring. J. Chem. Soc. Chem. Commun. 365-366. https://doi.org/10.1039/C39750000365
Dempsey, D., and Hook, I., 2000. Yew (Taxus) species-chemical and morphological variations. Pharm. Biol. 38, 274-280. https://doi.org/10.1076/1388-0209(200009)3841-AFT274
Ding, Q., Fang, D.M., Li, X.H. Gao, F., 2020. Two new taxane diterpenoids from Taxus baccata. Nat. Prod. Commun. 15. https://doi.org/10.1177/1934578X20953280
Dong, M., Zhang, M.L., Wang, Y.F., Zhang, X.P., Li, C.F., Shi, Q.W., Cong, B., Kiyota, H., 2007. Three new taxanes with the 10-alkoxy group from the heartwood of Taxus cuspidata. Biosci. Biotechnol. Biochem. 71, 70210-1 - 70210-4. https://doi.org/10.1271/bbb.70210
Doss, R.P., Carney, J.R., Shanks, C.H., Williamson, R.T., Chamberlain, J.D., 1997. Two new taxoids from European yew (Taxus baccata) that act as pyrethroid insecticide synergists with the black vine weevil (Otiorhynchus sulcatus). J. Nat. Prod. 60, 1130-1133. https://doi.org/10.1021/np9703353
Erdemoğlu, N., Şener, B., İde, S., 2001. Structural features of two taxoids from Taxus baccata L. growing in Turkey. J. Mol. Struct. 559, 227-233. https://doi.org/10.1016/S0022-2860(00)00722-5
Ettouati, L., Ahond, A., Poupat, C., Potier, P. 1991. Structural revision of taxine B, the main alkaloid of leaves of European yew, Taxus baccata. J. Nat. Prod. 54, 1455-1458.
Fang, W.S., Fang, Q.C., Liang, X.T., Lu, Y. and Zheng, Q.T., 1995. Taxachitrienes A and B, two new bicyclic taxane diterpenoids from Taxus chinensis. Tetrahedron 51, 8483-8490. https://doi.org/10.1016/0040-4020(95)00480-V
Fang, W.S., Fang, Q.C., Liang, X.T., 1996. Bicyclic taxoids from needles of Taxus chinensis. Planta Med. 62, 567-569. https://doi.org/10.1055/s-2006-957975
Fang, W., Fang, Q., Liang, X., Lu, Y., Wu, N., Zheng, Q., 1997. Taxuchin B, a new chlorine-containing taxoid. Chin. Chem. Lett. 8, 231-232.
Fedoreyev, S., Vasilevskaya, N., Veselova, M., Denisenko, V., Dmitrenok, P., 1998. A new C-14 oxygenated taxane from Taxus cuspidata cell culture. Fitoterapia 69, 430-432.
Fischedick, J.T., Johnson, S.R., Ketchum, R.E., Croteau, R.B. Lange, B.M., 2015. NMR spectroscopic search module for Spektraris, an online resource for plant natural product identification – Taxane diterpenoids from Taxus × media cell suspension cultures as a case study. Phytochemistry 113, 87-95. https://doi.org/10.1016/j.phytochem.2014.11.020
Fuji, K., Tanaka, K., Li, B., Shingu, T., Sun, H. Taga, T., 1992. Taxchinin A: a diterpenoid from Taxus chinensis. Tetrahedron Lett. 33, 7915-7916. https://doi.org/10.1016/S0040-4039(00)74777-0
Fuji, K., Tanaka, K., Li, B., Shingu, T., Yokoi, T., Sun, H. Taga, T., 1995. Structures of nine new diterpenoids from Taxus chinensis. Tetrahedron, 51, 10175-10188. https://doi.org/10.1016/0040-4020(95)00607-A
Fuji, K., Tanaka, K., Li, B., Shingu, T., Sun, H., Taga, T., 1993. Novel diterpenoids from Taxus chinensis. J. Nat. Prod. 56, 1520-1531. https://doi.org/10.1021/np50099a010
Fukushima, M., Takeda, J., Fukamiya, N., Okano, M., Tagahara, K., Zhang, S.X., Zhang, D.C., Lee, K.H., 1999. A new taxoid, 19-acetoxytaxagifine, from Taxus chinensis. J. Nat. Prod. 62, 140-142. https://doi.org/10.1021/np980202x
Gabetta, B., Peterlongo, F., Zini, G., Barboni, L., Rafaiani, G., Ranzuglia, P., Torregiani, E., Appendino, G., Cravotto, G., 1995a. Taxanes from Taxus x media. Phytochemistry 40, 1825-1828. https://doi.org/10.1016/0031-9422(95)00474-L
Gabetta, B., de Bellis, P., Pace, R., Appendino, G., Barboni, L., Torregiani, E., Gariboldi, P., Viterbo, D., 1995b. 10-Deacetylbaccatin III analogues from Taxus baccata. Journal of Natural Products. 1995 Oct;58(10):1508-14. https://doi.org/10.1021/np50124a005
Gabetta, B., Orsini, P., Peterlongo, F., and Appendino, G., 1998. Paclitaxel analogues from Taxus baccata. Phytochemistry 47, 1325-1329. https://doi.org/10.1016/S0031-9422(97)00712-7
Gabetta, B., Fuzzati, N., Orsini, P., Peterlongo, F., Appendino, G., Vander Velde, D.G., 1999. Paclitaxel analogues from Taxus × media cv. Hicksii. J. Nat. Prod. 62, 219-223. https://doi.org/10.1021/np9802264
Gao, Y.L., Zhou, J.Y., Ding, Y., Fang, Q.C., 1997. Chin. Chem. Lett. 8, 1057-1058.
Ge, G.B., Zhang, Y.Y., Hao, D.C., Hu, Y., Luan, H.W., Liu, X.B., He, Y.Q., Wang, Z.T. and Yang, L., 2008. Chemotaxonomic study of medicinal Taxus species with fingerprint and multivariate analysis. Planta Med. 74, 773-779. https://doi.org/10.1055/s-2008-1074531
Graf, E., and Berthold, H., 1957. Das amorphe und kristalline Taxin (Taxinalkaloide, 2. Mitt.). Pharm. Zent. 96, 385-395.
Graf, E., Kirfel, A., Wolff, G.-J., Breitmaier, E., 1982. Die Aufklärung von Taxin A aus Taxus baccata L. Liebigs Ann. Chem. 7, 376-381. https://doi.org/10.1002/jlac.198219820222
Graf, E., Weinand, S., Koch, B., Breitmaier, E., 1986. 13C-NMR-Untersuchung von Taxin B aus Taxus baccata L. Liebigs Ann Chem. 7, 1147-1151. https://doi.org/10.1002/jlac.198619860703
Guerra-Bubb, J., Croteau, R. Williams, R.M., 2012. The early stages of taxol biosynthesis: an interim report on the synthesis and identification of early pathway metabolites. Nat. Prod. Rep. 29, 683-696. https://doi.org/10.1039/C2NP20021J
Guerrero, B., Castillo, J., Aguilar, M.I., Delgado, G., 2000. 5a,7b, 9a,10b,13a-pentaacetoxy-4(20), 11-taxadiene (7b-acetoxy-Taxusin) and other constituents from the bark of the mexican yew, Taxus globosa (Taxaceae). J. Mex. Chem. Soc. 44, 148-150.
Gunawardana, G. P., Premachandran, U., Burres, N. S., Whittern, D. N., Henry, R., Spanton, S., McAlpine, J. B., 1992. Isolation of 9-dihydro-13-acetylbaccatin III from Taxus canadensis. J. Nat. Prod. 55, 1686-1689. https://doi.org/10.1021/np50089a022
Guo, Y., Diallo, B., Jaziri, M., Vanhaelen-Fastré, R., Vanhaelen, M., Ottinger, R., 1995a. Two new taxoids from the stem bark of Taxus baccata. J. Nat. Prod. 58, 1906-1912. https://doi.org/10.1021/np50126a017
Guo, Y., Vanhaelen-Fastré, R., Diallo, B., Vanhaelen, M., Jaziri, M., Homès, J., Ottinger, R., 1995b. Immunoenzymatic methods applied to the search for bioactive taxoids from Taxus baccata. J. Nat. Prod. 58, 1015-1023. https://doi.org/10.1021/np50121a005
Guo, Y., Diallo, B., Jaziri, M., Vanhaelen-Fastré, R., Vanhaelen, M., Ottinger, R., 1996. Immunological detection and isolation of a new taxoid from the stem bark of Taxus baccata. J. Nat. Prod. 59, 169-172. https://doi.org/10.1021/np960043m
Hai, P., Wen, S.Z., Li, Y., Gao, Y., Jiang, X.J., Wang, F., 2014. New taxane diterpenoids from Taxus yunnanensis. Nat. Prod. Bioprospect. 4, 47-51. https://doi.org/10.1007/s13659-014-0003-9
Hall, A.M., Tong, X.J., Chang, C.J., 1997. Taxoids from Taxus x media “Dark Green Spreader”. Nat. Prod. Lett. 10, 165-172. https://doi.org/10.1080/10575639708041190
Hao, D., Gu, X. and Xiao, P., 2015. Taxus medicinal resources. In Medicinal Plants: Chemistry, Biology and Omics, Woodhead Publishing, Cambridge, pp. 97-136.
Ho, T.I., Lin, Y.C., Lee, G.H., Peng, S.M., Yeh, M.K., Chen, F.C., 1987. Structure of taiwanxan. Acta Crystallogr. C 43, 1380-1382. https://doi.org/10.1107/S0108270187091807
Hosoyama, H., Inubushi, A., Katsui, T., Shigemori, H., Kobayashi, J.I., 1996. Taxuspines U, V, and W, new taxane and related diterpenoids from the Japanese yew Taxus cuspidata. Tetrahedron 52, 13145-13150. https://doi.org/10.1016/0040-4020(96)00777-6
Huang, C.O., Kingston, D.G., Magri, N.F., Samaranayake, G., Boettner, F.E., 1986. New taxanes from Taxus brevifolia, 2. J Nat Prod 49, 665-669. https://doi.org/10.1021/np50046a018
Huang, K., Liang, J., Gunatilaka, A.A.L., 1998. Studies on 1H-NMR of naturally occuring taxane diterpenoids and revising suggestions on some structures. Zhongguo Yaoke Daxue Xuebao 29, 259.
Huo, C., Su, X., Li, X., Zhang, X., Li, C., Wang, Y., Shi, Q., Kiyota, H., 2007a. Structural determination of a new 2(3→ 20)abeotaxane with an unusual 13β-substitution pattern and a new 6/8/6-ring taxane from Taxus cuspidata. Magn. Res. Chem. 45, 527-530. https://doi.org/10.1002/mrc.2001
Huo, C., Zhang, X., Li, C., Wang, Y., Shi, Q., Kiyota, H., 2007b. A new taxol analogue from the leaves of Taxus cuspidata. Biochem. Syst. Ecol. 35, 704-708. https://doi.org/10.1016/j.bse.2007.03.005
Huo, C.H., Wang, Y.F., Zhang, X.P., Li, C.F., Shi, Q.W., Kiyota, H., 2007c. A new metabolite with a new substitution pattern from the seeds of the Chinese yew, Taxus mairei. Chem. Biodivers. 4, 84-88. https://doi.org/10.1002/cbdv.200790009
Huo, C.H., Su, X.H., Wang, Y.F., Zhang, X.P., Shi, Q.W. Kiyota, H., 2007d. Canataxpropellane, a novel taxane with a unique polycyclic carbon skeleton (tricyclotaxane) from the needles of Taxus canadensis. Tetrahedron Lett. 48, 2721-2724. https://doi.org/10.1016/j.tetlet.2007.02.063
Ishiyama, H., Kakuguchi, Y., Arita, E., 2007. Taxezopidines O and P, new taxoids from Taxus cuspidata. Heterocycles 73, 341-348. https://doi.org/10.1002/chin.200819184
Jennewein, S., Rithner, C.D., Williams, R.M., Croteau, R.B., 2001. Taxol biosynthesis: taxane 13α-hydroxylase is a cytochrome P450-dependent monooxygenase. Proc. Natl. Acad. Sci. USA 98, 13595-13600. https://doi.org/10.1073/pnas.251539398
Jennewein, S., Rithner, C.D., Williams, R.M., Croteau, R., 2003. Taxoid metabolism: taxoid 14β-hydroxylase is a cytochrome P450-dependent monooxygenase. Archives of biochemistry and biophysics, 413(2), pp.262-270. https://doi.org/10.1016/S0003-9861(03)00090-0
Jennewein, S., Long, R.M., Williams, R.M., Croteau, R., 2004. Cytochrome P450 taxadiene 5α-hydroxylase, a mechanistically unusual monooxygenase catalyzing the first oxygenation step of taxol biosynthesis. Chem. Biol. 11, 379-387. https://doi.org/10.1016/j.chembiol.2004.02.022
Jenniskens, L.H., van Rozendaal, E.L., van Beek, T.A., Wiegerinck, P.H., Scheeren, H.W., 1996. Identification of six taxine alkaloids from Taxus baccata needles. J. Nat. Prod. 59, 117-123. https://doi.org/10.1021/np960033l
Jia, Z., and Zhang, Z., 1991. Taxanes from Taxus chinensis. Chin. Sci. Bull. 36, 1174-1177.
Ketchum, R.E.B., Tandon, M., Gibson, D.M., Begley, T., Shuler, M.L., 1999. Isolation of labeled 9-dihydrobaccatin III and related taxoids from cell cultures of Taxus canadensis elicited with methyl jasmonate. J. Nat. Prod. 62, 1395-1398. https://doi.org/10.1021/np990201k
Kingston, D.G., Hawkins, D.R., Ovington, L., 1982. New taxanes from Taxus brevifolia. J. Nat. Prod. 45, 466-470. https://doi.org/10.1021/np50022a019
Kingston, D.G., 2001. Taxol, a molecule for all seasons. Chem. Commun. 10, 867-880. https://doi.org/10.1039/B100070P
Kitagawa, I., Mahmud, T., Kobayashi, M., Shibuya, H., 1995. Taxol and its related taxoids from the needles of Taxus sumatrana. Chem. Pharm. Bull. 43, 365-367. https://doi.org/10.1248/cpb.43.365
Kiyota, H., Shi, Q.W., Oritani, T., Li, L., 2001. New 11(15→1)abeotaxane, 11(15→1),11(10→9)bisabeotaxane and 3,11-cyclotaxanes from Taxus yunnanensis. Biosci. Biotechnol. Biochem. 65, 35-40. https://doi.org/10.1271/bbb.65.35
Klettke, K.L., Sanyal, S., Mutatu, W., Walker, K.D., 2007. β-Styryl-and β-aryl-β-alanine products of phenylalanine aminomutase catalysis. JJ. Am. Chem. Soc. 129, 6988-6989. https://doi.org/10.1021/ja071328w
Kobayashi, J., Ogiwara, A., Hosoyama, H., Shigemori, H., Yoshida, N., Sasaki, T., Li, Y., Iwasaki, S., Naito, M., Tsuruo, T., 1994. Taxuspines A∼ C, new taxoids from Japanese yew Taxus cuspidata inhibiting drug transport activity of P-glycoprotein in multidrug-resistant cells. Tetrahedron 50, 7401-7416. https://doi.org/10.1016/S0040-4020(01)90470-3
Kobayashi, J.I., Inubushi, A., Hosoyama, H., Yoshida, N., Sasaki, T., Shigemori, H., 1995a. Taxuspines E∼ H and J, new taxoids from the Japanese yew Taxus cuspidata. Tetrahedron 51, 5971-5978. https://doi.org/10.1016/0040-4020(95)00255-7
Kobayashi, J., Hosoyama, H., Shigemori, H., Koiso, Y., Iwasaki, S., 1995b. Taxuspine D, a new taxane diterpene from Taxus cuspidata with potent inhibitory activity against Ca 2+-induced depolymerization of microtubules. Experientia 51, 592-595. https://doi.org/10.1007/BF02128750
Kobayashi, J.I., Hosoyama, H., Katsui, T., Yoshida, N., Shigemori, H., 1996. Taxuspines N, O, and P, new taxoids from Japanese yew, Taxus cuspidata. Tetrahedron 52, 5391-5396. https://doi.org/10.1016/0040-4020(96)00164-0
Kuo, W.L., Chen, F.C., Chen, K.J., Chen, J.J., 2015. Taxusumatrin, a new taxoid from the stem bark of Taxus sumatrana. Chem. Nat. Comp. 51, 427-430. https://doi.org/10.1007/s10600-015-1308-6
Kurono, M., Nakadaira, Y., Onuma, S., Sasaki, K., Nakanishi, K., 1963. Taxinine. Tetrahedron Lett. 4, 2153-2160. https://doi.org/10.1016/S0040-4039(01)90987-6
Lange, B.M., 2018. Commercial-scale tissue culture for the production of plant natural products: successes, failures and outlook. In Biotechnology of Natural Products, W. Schwab, B.M. Lange, and M. Wüst, Eds., Springer International Publishing, pp. 189-218. https://doi.org/10.1007/978-3-319-67903-7_8
Langley, B., Lythgoe, B., Scales, B., Scrowston, R., Trippett, S., Wray, D., 1962, 576. Taxine. Part II. Glycol cleavage fragments from O-cinnamoyltaxicin-I. J. Chem. Soc., 2972-2984. https://doi.org/10.1039/JR9620002972
Lei, X., Huang, S., Xiao, H., Gao, F., Zhou, X., 2018. A new taxane diterpenoid and a new neolignan from Taxus baccata. Nat. Prod. Commun. 13. https://doi.org/10.1177/1934578X1801301103
Li, Z., and Shi, Q., 2000. Studies on the chemical constituents of the bark of Taxus mairei. Zhong cao yao 31, 490-492.
Li, B., Tanaka, K., Fuji, K., Sun, H., Taga, T., 1993. Three new diterpenoids from Taxus chinensis. Chem. Pharm. Bull. 41, 1672-1673. https://doi.org/10.1248/cpb.41.1672
Li, S., Zhang, H., Yao, P., Sun, H., 1998. Two new taxoids from Taxus yunnanensis. Chin. Chem. Lett. 9, 1017.
Li, S.H., Zhang, H.J., Yao, P., Sun, H.D., Fong, H.H., 2000. Rearranged taxanes from the bark of Taxus yunnanensis. J. Nat. Prod. 63, 1488-1491. https://doi.org/10.1021/np000118t
Li, J., Davis, C.C., Del Tredici, P. and Donoghue, M.J., 2001. Phylogeny and biogeography of Taxus (Taxaceae) inferred from sequences of the internal transcribed spacer region of nuclear ribosomal DNA. Harvard Papers Bot. 6, 267-274. https://www.jstor.org/stable/41761651
Li, S., Zhang, H., Yao, P., Sun, H., Fong, H.H., 2001. Taxane diterpenoids from the bark of Taxus yunnanensis. Phytochemistry 58, 369-374. https://doi.org/10.1016/S0031-9422(01)00218-7
Li, S.H., Zhang, H.J., Niu, X.M., Yao, P., Sun, H.D., Fong, H.H., 2002. Taxuyunnanines S-V, new taxoids from Taxus yunnanensis. Planta Med. 68, 253-257. https://doi.org/ 10.1055/s-2002-23136
Li, S.H., Zhang, H.J., Niu, X.M., Yao, P., Sun, H.D., Fong, H.H., 2003. Novel taxoids from the Chinese yew Taxus yunnanensis. Tetrahedron 59, 37-45. https://doi.org/10.1016/S0040-4020(02)01483-7
Li, L., Cao, C., Huo, C., Zhang, M., Shi, Q., Kiyota, H., 2005a. Structure elucidation and complete NMR spectral assignments of a new taxane glycoside from the needles of Taxus cuspidata. Magn. Res. Chem. 43, 475-478. https://doi.org/10.1002/mrc.1579
Li, Z., Wang, C., Gu, J., Shi, Q., 2005b. Studies on chemical constituents in seeds of Taxus mairei II. Zhongguo Zhongyao Zazhi 30, 1260-1263.
Li, Z.P., Hu, C.H., Zhang, M.L., Shi, Q.W., 2006. Chemical constituents of seeds of Taxus mairei. Zhongcaoyao 37, 175-178.
Li, Y., Wang, Y.L., Ni, Z.Y., Dong, M., Cong, B., Sauriol, F., Zhang, M.L., Gu, Y.C., Shi, Q.W., Kiyota, H., 2011. A novel taxatetraene framework formed from a new natural taxane from Taxus cuspidata. Zeitschr. Naturforsch. B 66, 759-761. https://doi.org/10.1515/znb-2011-0721
Li, Y., Zhang, M.L., Wang, Y.F., Dong, M., Sauriol, F., Shi, Q.W., Kiyota, H., Gu, Y.C., Cong, B., 2012. Canataxpyran A, a novel 3,8-secotaxane with a 13,17-ether bridge from needles of Taxus canadensis. Heterocyc. Commun. 18, 57-60. https://doi.org/10.1515/hc-2012-0035
Li, Y., Ni, Z.Y., Guo, R., Wu, Y., Dong, M., Sauriol, F., Shi, Q., Gu, Y.C., Cong, B., 2013. A new taxane from the needles of Taxus cuspidata. Chem. Nat. Compd. 49, 277-280. https://doi.org/10.1007/s10600-013-0581-5
Li, N., Wang, J., Yan, H.M., Zhang, M.l., Shi, Q.W., Sauriol, F., Kiyota, H., Dong, M., 2015. Two new taxane-glycosides from the needles of Taxus canadensis. Z. Naturforsch. B 70, 829-835. https://doi.org/10.1515/znb-2015-0074
Lian, J.Y., Min, Z.D., Mizuno, M., Tanaka, T., Iinuma, M., 1988. Two taxane diterpenes from Taxus mairei. Phytochemistry 27, 3674-3675. https://doi.org/10.1016/0031-9422(88)80794-5
Liang, J., Min, Z., Niwa, M., 1988. Studies on the diterpenes of Taxus mairei. 2. Structure of 2-deacetoxytaxinine J. Huaxue Xuebao 46, 1053-1054.
Liang, J., and Kingston, D.G., 1993. Two new taxane diterpenoids from Taxus mairei. J. Nat. Prod. 56, 594-599. https://doi.org/10.1021/np50094a020
Liang, J.Y., Huang, K.S., Gunatilaka, A.L., 1998. A New 12-Deoxytaxane Diterpenoidfrom Taxus chinensis. Planta Med. 64, 187-188. https://doi.org/10.1055/s-2006-957403
Liang, J.Y., Yang, L., Cheng, Q.L., Zheng, F.M., Wei, X.L., Zhang, Y., 2002. Isolation and structural identification of taxanes in Taxus yunnanensis. Chin. Trad. Herb. Drugs. 33, 294-296.
Liu, C.L., Lin, Y.C., Lin, Y.M., Chen, F.C., 1984. Constituents of the heatwood of Taiwan yew. Tai-wan K'O Hsueh 38, 119.
Liu, W.C., Gong, T. Zhu, P., 2016. Advances in exploring alternative Taxol sources. R.S.C. Adv. 6, 48800-48809. https://doi.org/10.1039/C6RA06640B
Lucas, H., 1856. Ueber ein in den Blättern von Taxus baccata L. enthaltenes Alkaloid (das Taxin). Arch. Pharm. 135, 145-149. https://doi.org/10.1002/ardp.18561350203
Luh, L.J., El‐Razek, M.H.A., Liaw, C.C., Chen, C.T.A., Lin, Y.S., Kuo, Y.H., Chien, C.T., Shen, Y.C., 2009. Tri‐and bicyclic taxoids from the Taiwanese yew Taxus sumatrana. Helv. Chim. Acta 92, 1349-1358. https://doi.org/10.1002/hlca.200900022
Lun, B.S., Shao, L.W., Wang, Y., Li, C.C., Yu, H., Wang, C.H., Zhu, Y., 2017. Taxanes from Taxus wallichiana var. mairei cultivated in the southern area of the Yangtze River in China. Nat. Prod. Res. 31, 2341-2347. https://doi.org/10.1080/14786419.2017.1305381
Ma, W., Stahlhut, R.W., Adams, T.L., Park, G.L., Evans, W.A., Blumenthal, S.G., Gomez, G.A., Nieder, M.H., Hylands, P.J., 1994a. Yunnanxane and its homologous esters from cell cultures of Taxus chinensis var. mairei. J. Nat. Prod. 57, 1320-1324. https://doi.org/10.1021/np50111a027
Ma, W., Park, G.L., Gomez, G.A., Nieder, M.H., Adams, T.L., Aynsley, J.S., Sahai, O.P., Smith, R.J., Stahlhut, R.W., Hylands, P.J., 1994b. New bioactive taxoids from cell cultures of Taxus baccata. J. Nat. Prod. 57, 116-122. https://doi.org/10.1021/np50103a016
Mao, S., Chen, W., Liao, S., 1999. Studies on chemical constituents of the stem bark of Taxus cuspidata. Zhong Yao Cai 22, 346-347.
McElroy, C., and Jennewein, S. (2018). Taxol® Biosynthesis and Production: From Forests to Fermenters. In Biotechnology of Natural Products, W. Schwab, B.M. Lange, and M. Wüst, Eds., Springer International Publishing, pp. 145-185. https://doi.org/10.1007/978-3-319-67903-7_7
McLaughlin, J.L., Miller, R.W., Powell, R.G., Smith Jr, C.R., 1981. 19-Hydroxybaccatin III, 10-deacetylcephalomannine, and 10-deacetyltaxol: new antitumor taxanes from Taxus wallichiana. J. Nat. Prod. 44, 312-319. https://doi.org/10.1021/np50015a013
Miller, R.W., Powell, R.G., Smith Jr, C.R., Arnold, E., Clardy, J., 1981. Antileukemic alkaloids from Taxus wallichiana Zucc. J. Org. Chem. 46, 1469-1474. https://doi.org/10.1021/jo00320a045
Min, Z., Jiang, H., Liang, J., 1989. Studies on the taxane diterpenes of the heartwood from Taxus mairei. Acta Pharmacol. Sin. 24, 673-677.
Morita, H., Wei, L., Gonda, A., Takeya, K., Itokawa, H., 1997a. A taxoid from Taxus cuspidata var. nana. Phytochemistry 46, 583-586. https://doi.org/10.1016/S0031-9422(97)00308-7
Morita, H., Gonda, A., Wei, L., Yamamura, Y., Takeya, K., and Itokawa, H., 1997b. Taxuspinananes A and B, new taxoids from Taxus cuspidata var. nana. J. Nat. Prod. 60, 390-392. https://doi.org/10.1021/np9607159
Morita, H., Gonda, A., Wei, L., Yamamura, Y., Wakabayashi, H., Takeya, K., Itokawa, H., 1998a. Taxoids from Taxus cuspidata var. nana. Phytochemistry 48, 857-862. https://doi.org/10.1016/S0031-9422(97)00870-4
Morita, H., Gonda, A., Wei, L., Yamamura, Y., Wakabayashi, H., Takeya, K., Itokawa, H., 1998b. Four new taxoids from Taxus cuspidata var. nana. Planta Med. 64, 183-186. https://doi.org/10.1055/s-2006-957402
Morita, H., Machida, I., Hirasawa, Y., Kobayashi, J.I., 2005. Taxezopidines M and N, taxoids from the Japanese Yew, Taxus cuspidata. J. Nat. Prod. 68, 935-937. https://doi.org/10.1021/np0500880
Murakami, R., Shi, Q.W., Oritani, T., 1999a. A taxoid from the needles of the Japanese yew, Taxus cuspidata. Phytochemistry 52, 1577-1580. https://doi.org/10.1016/S0031-9422(99)00360-X
Murakami, R., Shi, Q.W., Horiguchi, T., Oritani, T., 1999b. A novel rearranged taxoid from needles of the Japanese yew, Taxus cuspidata Sieb. et Zucc. Biosci. Biotechnol. Biochem. 63, 1660-1663. https://doi.org/10.1271/bbb.63.1660
Nathorst, A.G., 1908. Palaeobotanische Mitteillungen. 7. Über Palyssya, StachyoTaxus und PaleoTaxus. K. Svenska Vetensk. Akad. Handl. 8, 1-16.
Nevarez, D.M., Mengistu, Y.A., Nawarathne, I.N. Walker, K.D., 2009. An N-aroyltransferase of the BAHD superfamily has broad aroyl CoA specificity in vitro with analogues of N-dearoylpaclitaxel. J. Am. Chem. Soc. 131, 5994-6002. https://doi.org/10.1021/ja900545m
Nguyen, N.T., Banskota, A.H., Tezuka, Y., Nobukawa, T., Kadota, S., 2003. Diterpenes and sesquiterpenes from the bark of Taxus yunnanensis. Phytochemistry 64, 1141-1147. https://doi.org/10.1016/S0031-9422(03)00503-X
Ni, Z.Y., Dong, M., Cong, B., Sauriol, F., Zhang, M.L., Wang, Y.F., Huo, C.H., Shi, Q.W., Kiyota, H., 2011. A novel taxane 13-glucoside and other taxanes from the leaves of Taxus cuspidata. Planta Med. 77, 281-283. https://doi.org/10.1055/s-0030-1250333
Oritani, T. and Sugiyama, T., 1999. Rearranged three 2(3->20)abeotaxanes and two 9(10->20)abeoabietanes from the bark of Chinese yew Taxus mairei. Nat. Prod. Lett. 13, 113-120. https://doi.org/10.1080/10575639908048831
Pan, Z.H., Zhang, M.L., Wang, Y.F., Dong, M., Huo, C.H., Sauriol, F., Shi, Q.W., Kiyota, H., and Zhang, Y.J., 2011. The first taxane peroxide from the rooted cuttings of Taxus canadensis. Heterocyc. Commun. 17, 87-91. https://doi.org/10.1515/hc.2011.019
Parmar, V.S., Jha, A., Bisht, K.S., Taneja, P., Singh, S.K., Kumar, A., Jain, R., Olsen, C.E., 1999. Constituents of the yew trees. Phytochemistry 50, 1267-1304. https://doi.org/10.1016/S0031-9422(98)00702-X
Petzke, T.L., Shi, Q.W., Sauriol, F., Mamer, O., Zamir, L.O., 2004. Taxanes from rooted cuttings of Taxus canadensis. J. Nat. Prod. 67, 1864-1869. https://doi.org/10.1021/np049836w
Poupat, C., Ahond, A., Potier, P., 1994. Nouveau taxoïde basique isolé des feuilles d'If, Taxus baccata: La 2-désacétyltaxine A. J. Nat. Prod. 57, 1468-1469. https://doi.org/10.1021/np50112a023
Prasain, J.K., Stefanowicz, P., Kiyota, T., Habeichi, F., Konishi, Y., 2001. Taxines from the needles of Taxus wallichiana. Phytochemistry 58, 1167-1170. https://doi.org/10.1016/S0031-9422(01)00305-3
Rao, K.V., Bhakuni, R.S., Hanuman, J.B., Davies, R., Johnson, J., 1996a. Taxanes from the bark of Taxus brevifolia. Phytochemistry 41, 863-866. https://doi.org/10.1016/0031-9422(95)00672-9
Rao, K.V., Reddy, G.C., Juchum, J., 1996b. Taxanes of the needles of Taxus x media. Phytochemistry 43, 439-442. https://doi.org/10.1016/0031-9422(96)00281-6
Rao, K.V., and Johnson, J.H., 1998. Occurrence of 2,6-dimethoxy cinnamaldehyde in Taxus floridana and structural revision of taxiflorine to taxchinin M. Phytochemistry 49, 1361-1364. https://doi.org/10.1016/S0031-9422(98)00089-2
Rao, K.V., and Juchum, J., 1998. Taxanes from the bark of Taxus brevifolia. Phytochemistry 47, 1315-1324. https://doi.org/10.1016/S0031-9422(97)00722-X
Sakai, J., Sasaki, H., Kosugi, K., 2001. Two taxoids from Taxus cuspidata as modulators of multidrag resistant tumor cells. Heterocycles 54, 999-1009. https://doi.org/10.3987/COM-00-S(I)68
Samaranayake, G., Magri, N.F., Jitrangsri, C. and Kingston, D.G., 1991. Modified taxols. 5. Reaction of taxol with electrophilic reagents and preparation of a rearranged taxol derivative with tubulin assembly activity. J. Org. Chem. 56, 5114-5119. https://doi.org/10.1021/jo00017a024
Sanchez-Muñoz, R., Perez-Mata, E., Almagro, L., Cusido, R.M., Bonfill, M., Palazon, J. Moyano, E., 2020. A novel hydroxylation step in the taxane biosynthetic pathway: a new approach to paclitaxel production by synthetic biology. Front. Bioeng. Biotechnol. 8, 410. https://doi.org/10.3389/fbioe.2020.00410
Schoendorf, A., Rithner, C.D., Williams, R.M., Croteau, R.B., 2001. Molecular cloning of a cytochrome P450 taxane 10β-hydroxylase cDNA from Taxus and functional expression in yeast. Proc. Natl. Acad. Sci. USA 98, 501-1506. https://doi.org/10.1073/pnas.98.4.1501
Sénilh, V., Blechert, S., Colin, M., Guénard, D., Picot, F., Potier, P., Varenne, P., 1984. Mise en evidence de nouveaux analogues du taxol extraits de Taxus baccata. J. Nat. Prod. 47, 131-137. https://doi.org/10.1021/np50031a019
Shen, Y.C., Tai, H.R., Chen, C.Y., 1996. New taxane diterpenoids from the roots of Taxus mairei. J. Nat. Prod. 59, 173-176. https://doi.org/10.1021/np960046z
Shen, Y.C. and Chen, C.Y., 1997. Taxanes from the roots of Taxus mairei. Phytochemistry 44, 1527-1533. https://doi.org/10.1016/S0031-9422(96)00748-0
Shen, Y.C., Chen, C.Y. Kuo, Y.H., 1998a. A new taxane diterpenoid from Taxus mairei. J. Nat. Prod. 61, 838-840. https://doi.org/10.1021/np970573y
Shen, Y.C., Chen, Y.J., Chen, C.Y., 1999a. Taxane diterpenoids from the seeds of Chinese yew Taxus chinensis. Phytochemistry 52, 1565-1569. https://doi.org/10.1016/S0031-9422(99)00310-6
Shen, Y.C., Chen, C.Y., Chen, Y.J., 1999b. Taxumairol M: a new bicyclic taxoid from seeds of Taxus mairei. Planta Med. 65, 582-584. https://doi.org/10.1055/s-2006-960833
Shen, Y.C., Lo, K.L., Chen, C.Y., and Kuo, Y.H., 2000a. New taxanes with an opened oxetane ring from the roots of Taxus mairei. J. Nat. Prod. 63, 720-722. https://doi.org/10.1021/np990629j
Shen, Y.C., Prakash, C.V., Hung, M.C., 2000b. Taxane diterpenoids from the root bark of Taiwanese Yew Taxus mairei. J. Chin. Chem. Soc. 47, 1125-1130. https://doi.org/10.1002/jccs.200000151
Shen, Y.C., Chen, C.Y. Hung, M.C., 2000c. Taxane diterpenoids from seeds of Taxus mairei. Chem. Pharm. Bull. 48, 1344-1346. https://doi.org/10.1248/cpb.48.1344
Shen, Y.C., Prakash, C.V., Chen, Y.J., Hwang, J.F., Kuo, Y.H., Chen, C.Y. 2001. Taxane diterpenoids from the stem bark of Taxus mairei. J. Nat. Prod. 64, 950-952. https://doi.org/10.1021/np010071r
Shen, Y.C., Chang, Y.T., Lin, Y.C., Lin, C.L., Kuo, Y.H., Chen, C.Y., 2002a. New taxane diterpenoids from the roots of Taiwanese Taxus mairei. Chem. Pharm. Bull. 50, 781-787. https://doi.org/10.1248/cpb.50.781
Shen, Y.C., Wang, S.S., Pan, Y.L., Lo, K.L., Chakraborty, R., Chien, C.T., Kuo, Y.H., Lin, Y.C., 2002b. New taxane diterpenoids from the leaves and twigs of Taxus sumatrana. J. Nat. Prod. 65, 1848-1852. https://doi.org/10.1021/np0202273
Shen, Y.C., Chang, Y.T., Wang, S.S., Lin, Y.C., Chen, C.Y., 2002c. Taxumairols X—Z, New Taxoids from Taiwanese Taxus mairei. Chemical and pharmaceutical bulletin 50, 1561-1565. https://doi.org/10.1248/cpb.50.1561
Shen, Y.C., Pan, Y.L., Lo, K.L., Wang, S.S., Chang, Y.T., Wang, L.T., Lin, Y.C., 2003. New taxane diterpenoids from Taiwanese Taxus sumatrana. Chem. Pharm. Bull. 51, 867-869. https://doi.org/10.1248/cpb.51.867
Shen, Y.C., Ko, C.L., Cheng, Y.B., Chiang, M.Y., Khalil, A.T., 2004. New regio-and stereoselective O-deacetylated and epoxy products of taxanes isolated from Taxus mairei. J. Nat. Prod. 67, 2136-2140. https://doi.org/10.1021/np040152y
Shen, Y.C., Lin, Y.S., Cheng, Y.B., Cheng, K.C., Khalil, A.T., Kuo, Y.H., Chien, C.T., and Lin, Y.C., 2005a. Novel taxane diterpenes from Taxus sumatrana with the first C-21 taxane ester. Tetrahedron 61, 1345-1352. https://doi.org/10.1016/j.tet.2004.10.110
Shen, Y.C., Hsu, S.M., Lin, Y.S., Cheng, K.C., Chien, C.T., Chou, C.H., Cheng, Y.B., 2005b. New bicyclic taxane diterpenoids from Taxus sumatrana. Chem. Pharm. Bull. 53, 808-810. https://doi.org/10.1248/cpb.53.808
Shen, Y.C., Cheng, K.C., Lin, Y.C., Cheng, Y.B., Khalil, A.T., Guh, J.H., Chien, C.T., Teng, C.M., and Chang, Y.T., 2005c. Three new taxane diterpenoids from Taxus sumatrana. J. Nat. Prod. 68, 90-93. https://doi.org/10.1021/np040132w
Shen, Y.C., Lin, Y.S., Hsu, S.M., Khalil, A.T., Wang, S.S., Chien, C.T., Kuo, Y.H., and Chou, C.H., 2007. Tasumatrols P–T, five new taxoids from Taxus sumatrana. Helv. Chim. Acta 90, 1319-1329. https://doi.org/10.1002/hlca.200790133
Shi, Q.W., Oritani, T., Kiyota, H. 1998a. 14 β-HydroxyTaxusin, a New Taxane from Taxus Mairei. Nat. Prod. Lett. 12, 85-90. https://doi.org/10.1080/10575639808048274
Shi, Q.W., Oritani, T., Sugiyama, T., Kiyota, H., 1998b. Two new taxanes from Taxus chinensis var. mairei. Planta Med. 64, 766-769. https://doi.org/10.1055/s-2006-957580
Shi, Q.W., Oritani, T., Kiyota, H., Horiguchi, T., 1998c. Isolation and structure elucidation of a new 11(15→1)-abeotaxane from the Chinese yew, Taxus mairei. Nat. Prod. Lett. 12, 67-74. https://doi.org/10.1080/10575639808048873
Shi, Q.W., Oritani, T., Sugiyama, T., 1998d. A newly rearranged 2(3→20)abeotaxane diterpene from the bark of Chinese yew, Taxus mairei. Biosci. Biotechnol. Biochem. 62, 2263-2266. https://doi.org/10.1271/bbb.62.2263
Shi, Q.W., Oritani, T., Sugiyama, T., Kiyota, H., 1998e. Three novel bicyclic 3,8-secotaxane diterpenoids from the needles of the Chinese yew, Taxus chinensis var. mairei. J. Nat. Prod. 61, 1437-1440. https://doi.org/10.1021/np980213q
Shi, Q.W., Oritani, T., Sugiyama, T., Cheng, Q., 1999a. Two novel taxane diterpenoids from the seeds of Chinese yew, Taxus mairei. Nat. Prod. Lett. 13, 305-312. https://doi.org/10.1080/10575639908048802
Shi, Q.W., Oritani, T., Sugiyama, T., Zhao, D., Murakami, R., 1999b. Three new taxane diterpenoids from seeds of the Chinese yew, Taxus yunnanensis and T. chinensis var. mairei. Planta Med. 65, 767-770. https://doi.org/10.1055/s-2006-960864
Shi, Q.W., Oritani, T., Sugiyama, T., Yamada, T., 1999c. Taxane diterpenoids from the seeds of Chinese yew, Taxus mairei. Nat. Prod. Lett. 13, 179-186. https://doi.org/10.1080/10575639908048784
Shi, Q.W., Oritani, T., Sugiyama, T., Yamada, T., 1999d. Two novel pseudoalkaloid taxanes from the Chinese yew, Taxus chinensis var. mairei. Phytochemistry. 52, 1571-1575. https://doi.org/10.1016/S0031-9422(99)00359-3
Shi, Q.W., Oritani, T., Horiguchi, T., Sugiyama, T., Murakami, R., Yamada, T. 1999e. Four novel taxane diterpenoids from the needles of Japanese yew, Taxus cuspidata. Biosci. Biotechnol. Biochem. 63, 924-929. https://doi.org/10.1271/bbb.63.924
Shi, Q.W., Oritani, T., Sugiyama, T., Murakami, R., and Wei, H.Q., 1999f. Six new taxane diterpenoids from the seeds of Taxus chinensis var. mairei and Taxus yunnanensis. J. Nat. Prod. 62, 1114-1118. https://doi.org/10.1021/np990106b
Shi, Q.W., Oritani, T., Sugiyama, T., Horiguchi, T., Murakami, R., Zhao, D., Oritani, T., 1999g. Three new taxane diterpenoids from the seeds of Taxus yunnanensis Cheng et L.K. Fu and T. cuspidata Sieb et Zucc. Tetrahedron 55, 8365-8376. https://doi.org/10.1016/S0040-4020(99)00466-4
Shi, Q.W., Oritani, T., Sugiyama, T., Murakami, R., Yamada, T., 1999h. Two new taxane diterpenoids from the seeds of the Chinese yew, Taxus yunnanensis. J. Asian Nat. Prod. Res. 2, 71-79. https://doi.org/10.1080/10286029908039894
Shi, Q.W., Pritani, T., Sugiyama, T., Liyota, H., Horiguchi, T., 1999i. Three new rearranged taxane diterpenoids from the bark of Taxus chinensis var. mairei and the needles of Taxus cuspidata. Heterocycles 51, 841-850. https://doi.org/10.3987/COM-98-8446
Shi, Q.W., Oritani, T., Sugiyama, T., 1999j. A new 11 (15→1)-abeotaxane and two abietane diterpenoids from the bark of Taxus mairei. Nat. Prod. Lett. 13, 105-112. https://doi.org/10.1080/10575639908048830
Shi, Q.W., Oritani, T., Sugiyama, T., 1999k. Three rearranged 2(3→20)abeotaxanes from the bark of Taxus mairei. Phytochemistry 52, 1559-1563. https://doi.org/10.1016/S0031-9422(99)00233-2
Shi, Q.W., Oritani, T., Sugiyama, T., Yamada, T., 1999l. Two novel bicyclic 3,8-secotaxoids from the needles of Taxus mairei. Nat. Prod. Lett. 13, 171-178. https://doi.org/10.1080/10575639908048783
Shi, Q.W., Oritani, T., Sugiyama, T., Murakami, R., Horiguchi, T., 1999m. Three new bicyclic taxane diterpenoids from the needles of Japanese yew, Taxus cuspidata Sieb. et Zucc. J. Asian Nat. Prod. Res. 2, 63-70. https://doi.org/10.1080/10286029908039893
Shi, Q.W., Oritani, T., Sugiyama, T., Yamada, T., 1999n. Isolation and structural determination of a novel bicyclic taxane diterpene from needles of the Chinese yew, Taxus mairei. Biosci. Biotechnol. Biochem. 63, 756-759. https://doi.org/10.1271/bbb.63.756
Shi, Q.W., Oritani, T., and Sugiyama, T. 1999o. Two bicyclic taxane diterpenoids from the needles of Taxus mairei. Phytochemistry 50, 633-636. https://doi.org/10.1016/S0031-9422(98)00563-9
Shi, Q.W., Oritani, T., Sugiyama, T., 1999p. Three novel bicyclic taxane diterpenoids with verticillene skeleton from the needles of Chinese yew, Taxus chinensis var. mairei. Planta Med. 65, 356-359. http://doi.org/10.1055/s-1999-14002
Shi, Q.W., Oritani, T., Sugiyama, T., 1999q. Bicyclic taxane with a verticillene skeleton from the needles of Chinese yew, Taxus mairei. Nat. Prod. Lett. 13, 81-88. https://doi.org/10.1080/10575639908048827
Shi, Q.W., and Oritani, T., 2000. Three new 2(3→20)-abeo-taxoids from the bark of the Chinese yew, Taxus mairei. Nat. Prod. Lett. 14, 273-280. https://doi.org/10.1080/10575630008041242
Shi, Q.W., Oritani, T., Gu, J.S., Meng, Q.Z., Liu, R.L., 2000a. Three new taxane diterpenoids from the seeds of the Chinese yew, Taxus chinensis var. mairei. J. Asian Nat. Prod. Res. 2, 311-319. https://doi.org/10.1080/10286020008041371
Shi, Q.W., Oritani, T., Kiyota, H., Zhao, D., 2000b. Taxane diterpenoids from Taxus yunnanensis and Taxus cuspidata. Phytochemistry 54, 829-834. https://doi.org/10.1016/S0031-9422(00)00186-2
Shi, Q.W., Oritani, T., Zhao, D., Murakami, R., Oritani, T., 2000c. Three new taxoids from the seeds of Japanese yew, Taxus cuspidata. Planta Med. 66, 294-299. https://doi.org/10.1055/s-2000-8565
Shi, Q.W., Oritani, T., Sugiyama, T., and Oritani, T., 2000d. Three new taxane diterpenoids from the seeds of the Japanese yew, Taxus cuspidata. Nat. Prod. Lett. 14, 265-272. https://doi.org/10.1080/10575630008041241
Shi, Q.W., Oritani, T., Zhao, D., 2000e. New taxane diterpenoid from seed of the Chinese yew, Taxus yunnanensis. Bioschi. Biotechnol. Biochem. 64, 869-872. https://doi.org/10.1271/bbb.64.869
Shi, Q.W., Oritani, T., Kiyota, H., Murakami, R., 2001. Three new taxoids from the leaves of the Japanese yew, Taxus cuspidata. Nat. Prod. Lett. 15, 55-62. ttps://doi.org/10.1080/10575630108041258
Shi, Q.W., Sauriol, F., Mamer, O., Zamir, L.O. 2002. A novel minor metabolite (taxane?) from Taxus canadensis needles. Tetrahedron Lett. 43, 6869-6873. https://doi.org/10.1016/S0040-4039(02)01535-6
Shi, Q.W., Sauriol, F., Mamer, O., Zamir, L.O., 2003a. New minor taxane derivatives from the needles of Taxus canadensis. J. Nat. Prod. 66, 1480-1485. https://doi.org/10.1021/np030322r
Shi, Q.W., Sauriol, F., Mamer, O., Zamir, L.O., 2003b. New taxanes from the needles of Taxus canadensis. J. Nat. Prod. 66, 470-476. https://doi.org/10.1021/np020361n
Shi, Q.W., Petzke, T.L., Sauriol, F., Mamer, O., Zamir, L.O., 2003c. Taxanes in rooted cuttings vs. mature Japanese yew. Can. J. Chem. 81, 64-74. https://doi.org/10.1139/v02-190
Shi, Q.W., Sauriol, F., Mamer, O., Zamir, L.O., 2003d. New minor taxane analogues from the needles of Taxus canadensis. Bioorg. Med. Chem. 11, 293-303. https://doi.org/10.1016/S0968-0896(02)00347-4
Shi, Q.W., Sauriol, F., Mamer, O., Zamir, L.O. 2003e. First example of a taxane-derived propellane in Taxus canadensis needles. Chem. Commun., 68-69. https://doi.org/10.1039/B209312J
Shi, Q.W., Nikolakakis, A., Sauriol, F., Mamer, O., Zamir, L.O. 2003f. Canadensenes: Natural and semi-synthetic analogues. Can. J. Chem. 81, 406-411. https://doi.org/10.1139/v03-084
Shi, Q.W., Lederman, Z., Sauriol, F., McCollum, R.S., Zamir, L.O., 2004a. A yew in Israel, new taxane derivatives. J. Nat. Prod. 67, 168-173. https://doi.org/10.1021/np030311y
Shi Q.W., Ji, X., Lesimple, A., Sauriol, F., Zamir, L.O., 2004b. Taxanes with C-5-amino-side chains from the needles of Taxus canadensis. Phytochemistry. 2004 65, 3097-3106. https://doi.org/10.1016/j.phytochem.2004.08.042
Shi, Q.W., Li, Z.P., Zhao, D., Gu, J.S., Oritani, T. Kiyota, H., 2004c. New 2(3→20)abeotaxane and 3,11-cyclotaxane from needles of Taxus cuspidata. Biosci. Biotechnol. Biochem. 68, 1584-1587. https://doi.org/10.1271/bbb.68.1584
Shi, Q.W., Sauriol, F., Lesimple, A., Zamir, L.O., 2004d. First three examples of taxane-derived di-propellanes in Taxus canadensis needles. Chem. Commun., 544-545. https://doi.org/10.1039/B316051C
Shi, Q.W., Sauriol, F., Park, Y., Smith Jr, V., Lord, G., Zamir, L.O., 2005a. First example of conformational exchange in a natural taxane enolate. Magn. Res. Chem. 43, 798-804. https://doi.org/10.1002/mrc.1630
Shi, Q.W., Li, L.G., Li, Z.P., Cao, C.M., Kiyota, H., 2005b. A novel 3,8-seco-taxane metabolite from the seeds of the Chinese yew, Taxus mairei. Tetrahedron Lett. 46, 6301-6303. https://doi.org/10.1016/j.tetlet.2005.07.040
Shi, Q.W., Li, Z.P., Zhao, D., Gu, J.S., Kiyota, H., 2006a. Isolation and structure revision of 10-deacetyltaxinine from the seeds of the Chinese yew, Taxus mairei. Nat Prod. Res. 20, 47-51. ttps://doi.org/10.1080/14786410500111184
Shi, Q.W., Cao, C.M., Gu, J.S., Kiyota, H., 2006b. Four new epoxy taxanes from needles of Taxus cuspidata (Taxaceae). Nat. Prod. Res. 20, 173-179. https://doi.org/10.1080/14786410500049426
Shi, Q.W., Zhao, Y.M., Si, X.T., Li, Z.P., Yamada, T., Kiyota, H., 2006c. 1-Deoxypaclitaxel and abeo-taxoids from the seeds of Taxus mairei. J. Nat. Prod. 69, 280-283. https://doi.org/10.1021/np0503451
Shi, Q.W., Si, X.T., Zhao, Y.M., Su, X.H., Li, X., Zamir, L.O., Yamada, T., Kiyota, H., 2006d. Two new alkaloidal taxoids from the needles of Taxus canadensis. Biosci. Biotechnol. Biochem. 70, 732-736. https://doi.org/10.1271/bbb.70.732
Shi, Q.W., Dong, M., Huo, C.H., Su, X.H., Li, C.F., Zhang, X.P., Wang, Y.F., Kiyota, H., 2007. New 14-hydroxy-taxane and 2α,20-epoxy-11(15→1)abeotaxane from the needles of Taxus canadensis. Biosci. Biotechnol. Biochem. 71, 1777-1780. https://doi.org/10.1271/bbb.70063
Shigemori, H., Wang, X.X., Yoshida, N., Kobayashi, J.I., 1997. Taxuspines X-Z, new taxoids from Japanese yew, Taxus cuspidata. Chem. Pharm. Bull. 45, 1205-1208. https://doi.org/10.1248/cpb.45.1205
Shigemori, H., Sakurai, C.A., Hosoyama, H., Kobayashi, A., Kajiyama, S., Kobayashi, J.I., 1999. Taxezopidines J, K, and L, new taxoids from Taxus cuspidata inhibiting Ca2+-induced depolymerization of microtubules. Tetrahedron 55, 2553-2558. https://doi.org/10.1016/S0040-4020(99)00052-6
Shinozaki, Y., Fukamiya, N., Fukushima, M., Okano, M., Nehira, T., Tagahara, K., Zhang, S.X., Zhang, D.C., and Lee, K.H., 2001. DanTaxusins A and B, two new taxoids from Taxus yunnanensis. J. Nat. Prod. 64, 1073-1076. https://doi.org/10.1021/np0100643
Shinozaki, Y., Fukamiya, N., Fukushima, M., Okano, M., Nehira, T., Tagahara, K., Zhang, S.X., Zhang, D.C., and Lee, K.H., 2002. DanTaxusins C and D, two novel taxoids from Taxus yunnanensis. J. Nat. Prod. 65, 371-374. https://doi.org/10.1021/np010435f
Shrestha, T.B., Chetri, S.K.K., Banskota, A.H., Manandhar, M.D., Taylor, W.C., 1997. 2-Deacetoxytaxinine B: A new taxane from Taxus wallichiana. J. Nat. Prod. 60, 820-821. https://doi.org/10.1021/np9606153
Soto, J., and Castedo, L., 1998. Taxoids from european yew, Taxus baccata L. Phytochemistry 47, 817-819. https://doi.org/10.1016/S0031-9422(97)00619-5
Soto, J., Fuentes, M., Castedo, L., 1996. Teixidol, an abeo-taxane from European yew, Taxus baccata. Phytochemistry 43, 313-314. https://doi.org/10.1016/0031-9422(96)00246-4
Spjut, R.W., 2007. Taxonomy and nomenclature of Taxus (Taxaceae). J. Bot. Res. Inst. Texas, 203-289. https://www.jstor.org/stable/41971409
Srividya, N., Lange, I., Hartmann, M., Li, Q., Mirzaei, M. Lange, B.M., 2020. Biochemical characterization of acyl activating enzymes for side chain moieties of Taxol and its analogs. J. Biol. Chem. 295, 4963-4973. https://doi.org/10.1074/jbc.RA120.012663
Sugiyama, T., Oritani, T., Oritani, T., 1994. A novel taxane diterpenoid from Japanese yew, Taxus cuspidata. BBiosci. Biotechnol. Biochem. 58, 1923-1924. https://doi.org/10.1271/bbb.58.1923
Sun, Z.H., Chen, Y., Guo, Y.Q., Qiu, J., Zhu, C.G., Jin, J., Tang, G.H., Bu, X.Z., Yin, S., 2015. Isolation and cytotoxicity evaluation of taxanes from the barks of Taxus wallichiana var. mairei. Bioorg. Med. Chem. Lett. 25, 1240-1243. https://doi.org/10.1016/j.bmcl.2015.01.056
Tanaka, K., Fuji, K., Yokoi, T., Shingo, T., Li, B., and Sun, H., 1994. On the structures of six new diterpenoids, taxchinins E, H, I, J, K and taxchin B. Chem. Pharm. Bull. 42, 1539-1541. https://doi.org/10.1248/cpb.42.1539
Tanaka, K., Fuji, K., Yokoi, T., Shingu, T., Li, B., Sun, H., 1996. Structures of taxchinins L and M, two new diterpenoids from Taxus chinensis var. mairei. Chem. Pharm. Bull. 44, 1770-1774. https://doi.org/10.1248/cpb.44.1770
Tong, X., Fang, W., Zhou, J., He, C., 1993. Studies on the chemical constituents of Taxus cuspidata. Chin. Chem. Lett. 4, 887-887.
Tong, X.J., Fang, W.S., Zhou, J.Y., He, C.H., Chen, W.M., Fang, Q.C., 1995. Three new taxane diterpenoids from needles and stems of Taxus cuspidata. J. Nat. Prod. 58, 233-238. https://doi.org/10.1021/np50116a011
Topcu, G., Sultana, N., Akhtar, F., Habib-ur-rehman, Hussain, T., Choudhary, M.I., Atta-ur-rahman, 1994. Taxane diterpenes from Taxus baccata. Nat. Prod. Lett. 4, 93-100. https://doi.org/10.1080/10575639408044919
van der Velde, D.G., Georg, G.I., Gollapudi, S.R., Jampani, H.B., Liang, X.Z., Mitscher, L.A., and Ye, Q.M., 1994. Wallifoliol, a taxol congener with a novel carbon skeleton, from Himalayan Taxus wallichiana. J. Nat. Prod. 57, 862-867. https://doi.org/10.1021/np50108a032
Verdian-Rizi, M., Hadjiakhoondi, A., Rezazadeh, S., Khanavi, M., Pirali-Hamedani, M., 2008. Taxuspinanane G from the aerial parts of Taxus baccata L. growing in Iran. J. Med. Plants 4, 120-124.
Vidensek, N., Lim, P., Campbell, A., Carlson, C., 1990. Taxol content in bark, wood, root, leaf, twig, and seedling from several Taxus species. J. Nat. Prod. 53, 1609-1610. https://doi.org/10.1021/np50072a039
Viterbo, D., Milanesio, M.A., Appendino, G., Chattopadhyay, S.K., Saha, G.C., 1997. 1-hydroxybaccatin I, C32H44O14, and 2-deacetoxydecinnamoyltaxinine J, C28H40O9. Acta Crystallogr. C: Crystal Struct. Commun. 53, 1687-1690. https://doi.org/10.1107/S0108270197009256
Walker, K., Schoendorf, A., Croteau, R., 2000. Molecular cloning of a taxa-4(20),11(12)-dien-5α-ol-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli. Arch. Biochem. Biophys. 374, 371-380. https://doi.org/10.1006/abbi.1999.1609
Walker, K. and Croteau, R., 2000a. Taxol biosynthesis: molecular cloning of a benzoyl-CoA: taxane 2α-O-benzoyltransferase cDNA from Taxus and functional expression in Escherichia coli. Proc. Natl. Acad. Sci. USA 97, 13591-13596. https://doi.org/10.1073/pnas.250491997
Walker, K. and Croteau, R., 2000b. Molecular cloning of a 10-deacetylbaccatin III-10-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli. Proc. Natl. Acad. Sci. USA 97, 583-587. https://doi.org/10.1073/pnas.97.2.583
Walker, K., Long, R. Croteau, R., 2002a. The final acylation step in taxol biosynthesis: cloning of the taxoid C13-side-chain N-benzoyltransferase from Taxus. Proc. Natl. Acad. SCi. USA 99, 9166-9171. https://doi.org/10.1073/pnas.082115799
Walker, K., Fujisaki, S., Long, R., Croteau, R., 2002b. Molecular cloning and heterologous expression of the C-13 phenylpropanoid side chain-CoA acyltransferase that functions in Taxol biosynthesis. Proc. Natl. Acad. Sci. USA 99, 12715-12720. https://doi.org/10.1073/pnas.192463699
Walker, K.D., Klettke, K., Akiyama, T. Croteau, R., 2004. Cloning, heterologous expression, and characterization of a phenylalanine aminomutase involved in Taxol biosynthesis. J. Biol. Chem. 279, 53947-53954. https://doi.org/10.1074/jbc.M411215200
Wang, X.X., Shigemori, H., Kobayashi, J.I., 1996a. Taxuspines Q, R, S, and T, new taxoids from Japanese yew Taxus cuspidata. Tetrahedron 52, 12159-12164. https://doi.org/10.1016/0040-4020(96)00706-5
Wang, X.X., Shigemori, H., and Kobayashi, J.I., 1996b. Taxuspines K, L, and M, new taxoids from Japanese yew Taxus cuspidata. Tetrahedron 52, 2337-2342. https://doi.org/10.1016/0040-4020(95)01074-2
Wang, X.X., Shigemori, H., Kobayashi, J.I. 1997. Taxezopidine A, a novel taxoid from seeds of Japanese yew Taxus cuspidata. Tetrahedron Lett. 38, 7587-7588. https://doi.org/10.1016/S0040-4039(97)01789-9
Wang, X.X., Shigemori, H., and Kobayashi, J.I., 1998. Taxezopidines B−H, new taxoids from Japanese yew Taxus cuspidata. J. Nat. Prod. 61, 474-479. https://doi.org/10.1021/np970584r
Wang, F., Peng, L.Y., Zhao, Y., Gu, K., Zhao, Q.S., Sun, H.D., 2003a. A new taxoid from Taxus chinensis. Yunnan Zhiwu Yanjiu 25, 507.
Wang, F., Liyan, P., Yu, Z., Kun, G., Qinshi, Z., Handong, S., 2003b. Taxoids from the leaves and stems of Taxus chinensis. Acta Bot. Yunnan. 25, 369-376.
Wang, F.S., Peng, L.Y., Zhao, Y., Xu, G., Zhao, Q.S., Sun, H.D., 2004a. Three new oxetane-ring-containing taxoids from Taxus c hinensis. J. Nat. Prod. 67, 905-907. https://doi.org/10.1021/np034042n
Wang, F., Peng, L., Zhao, Y., Zhao, Q., Gu, K., Sun, H., 2004b. A new taxoid from leaves and branches of Taxus chinensis. Chin. Chem. Lett. 15, 307-308.
Wang, C.L., Zhang, M.L., Cao, C.M., Shi, Q.W., Kiyota, H., 2005. First example of 11,12-epoxytaxane-glucoside from the needles of Taxus cuspidata. Heterocyc. Commun. 11, 211-214. https://doi.org/10.1515/HC.2005.11.3-4.211
Wang, L., Bai, L., Tokunaga, D., Watanabe, Y., Hasegawa, T., Sakai, J.-i., Tang, W., Bai, Y., Hirose, K., Yamori, T., 2008. The polar neutral and basic taxoids isolated from needles and twigs of Taxus cuspidata and their biological activity. J. Wood Sci. 54, 390-401. https://doi.org/10.1007/s10086-008-0964-6
Wang, Y.F., Shi, Q.-W., Dong, M., Kiyota, H., Gu, Y.-C., and Cong, B., 2011. Natural taxanes: developments since 1828. Chem. Rev. 111, 7652-7709. https://doi.org/10.1021/cr100147u
Wang, L.Y., Ding, L.M., Huo, S.C., Sun, L., Sun, Z., 2013. Three new taxoids from the seed of Taxus cuspidata. J. Nat. Med. 67, 827-832. https://doi.org/10.1007/s11418-012-0731-2
Wang, Y., Wang, J., Wang, H., Ye, W., 2016. Novel taxane derivatives from Taxus wallichiana with high anticancer potency on tumor cells. Chem. Biol. Drug Design 88, 556-561. https://doi.org/10.1111/cbdd.12782
Wani, M.C., Taylor, H.L., Wall, M.E., Coggon, P., McPhail, A.T., 1971. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 93, 2325-2327. https://doi.org/10.1021/ja00738a045
Wiedenfeld, H., Knoch, F., Zhang, Z.P., 1995. 2α,5α,9α-Trihydroxy-10β,13α-diacetoxy-4β,20-epoxy-taxa-11-en, ein neues Taxan aus Taxus chinensis. Acta Crystallogr. C 51, 2184-2186. https://doi.org/10.1107/S010827019500429X
Witherup, K.M., Look, S.A., Stasko, M.W., Ghiorzi, T. J., Muschik, G.M., Cragg, G.M., 1990. Taxus spp. needles contain amounts of taxol comparable to the bark of Taxus brevifolia: analysis and isolation. J. Nat. Prod. 53, 1249-1255. https://doi.org/10.1021/np50071a017
Woods, M.C., Chiang, H.-C., Nakadaira, Y., Nakanishi, K., 1968. Nuclear Overhauser effect, a unique method of defining the relative stereochemistry and conformation of taxane derivatives. J. Am. Chem. Soc. 90, 522-523. https://doi.org/10.1021/ja01004a074
Xia, Z.H., Peng, L.Y., Zhao, Y., Xu, G., Zhao, Q.S., Sun, H.D. 2005a. Two new taxoids from Taxus chinensis. Chem. Biodiv. 2, 1316-1319.
https://doi.org/10.1002/cbdv.200590103
Xia, Z.H., Peng, L.Y., Li, R.T., Zhao, Q.S., and Sun, H.D., 2005b. Two new taxoids from the needles and stems of Taxus chinensis. Heterocycles 65, 1404-1408. https://doi.org/10.3987/COM-04-10305
Xiao, C., Zhou, J.Y., Chen, W.M., Lu, Y., Zheng, Q.T., 1994. Structure identification of a new component from stems and leaves of Taxus yunnanensis. Acta Pharm. Sin. 29, 355.
Xu, X.H., Sun, B.N., Yan, D.F., Wang, J. Dong, C., 2015. A Taxus leafy branch with attached ovules from the Lower Cretaceous of Inner Mongolia, North China. Cretaceous Res. 54, 266-282. https://doi.org/10.1016/j.cretres.2014.12.014
Yang, S.J., Fang, J.M., Cheng, Y.S., 1996. Taxanes from Taxus mairei. Phytochemistry 43, 839-842. https://doi.org/10.1016/0031-9422(96)00375-5
Yang, S.J., Fang, J.M., Cheng, Y.S., 1999. Abeo-taxanes from Taxus mairei. Phytochemistry 50, 127-130. https://doi.org/10.1016/S0031-9422(98)00458-0
Yang, C., Wang, J.S., Luo, J.G., Kong, L.Y., 2009. A pair of taxoids from the needles of Taxus canadensis. J. Asian Nat. Prod. Res. 11, 534-538. https://doi.org/10.1080/10286020902932690
Yang, C.P.H., and Horwitz, S.B., 2017. Taxol®: the first microtubule stabilizing agent. Int. J. Mol. Sci. 18, 1733. https://doi.org/10.3390/ijms18081733
Yao, G.D., Zhang, H.F., Huang, Y., Sauriol, F., Shi, Q.W., Kiyota, H., Gu, Y.C., 2013. A New taxane with a 4β,20-epoxy ring from the rooted cuttings of Taxus canadensis. Chem. Nat. Compd. 49, 861-863. https://doi.org/10.1007/s10600-013-0765-z
Yeh, M.K., Wang, J.S., Liu, L.P., Chen, F.C., 1988. A new taxane derivative from the heartwood of Taxus mairei. Phytochemistry 27, 1535-1536. https://doi.org/10.1016/0031-9422(88)80234-6
Yoshizaki, F., FukudaA, M., HisamichiI, S. Ishida, T., 1988. Structures of taxane diterpenoids from the seeds of Japanese yew, Taxus cuspidata. Chem. Pharm. Bull. 36, 2098-2102. https://doi.org/10.1248/cpb.36.2098
Yu, S.H., Ni, Z.Y., Zhang, J., Dong, M., Sauriol, F., Huo, C.H., Shi, Q.W., Gu, Y.C., Kiyota, H., Cong, B., 2009. Taxusecone, a novel taxane with an unprecedented 11,12-secotaxane skeleton, from Taxus cuspidata needles. Biosci. Biotechnol. Biochem. 73, 1-3. https://doi.org/10.1271/bbb.80698
Yue, Q., Fang, Q.C., Liang, X.T., He, C.H., 1995a. Taxayuntin E and F: Two taxanes from leaves and stems of Taxus yunnanensis. Phytochemistry 39, 871-873. https://doi.org/10.1016/0031-9422(95)00017-2
Yue, Q., Fang, Q., Liang, X., 1995b. Taxayuntin G: a new taxoid from Taxus yunnanensis. Chin. Chem. Lett. 6, 225-228.
Yue, Q., Fang, Q.C., Liang, X.T., He, C.H., Jing, X.L., 1995c. Rearranged taxoids from Taxus yunnanensis. Planta Med. 61, 375-377. https://doi.org/10.1021/np000118t
Yue, Q., Fang, Q.C., Liang, X.T., 1996. A taxane-11,12-oxide from Taxus yunnanensis. Phytochemistry 43, 639-642. https://doi.org/10.1016/0031-9422(96)00299-3
Zamir, L.O., Nedea, M.E., Bélair, S., Sauricol, F., Mamer, O., Jacqmain, E., Jean, F.I., Garneau, F.X., 1992. Taxanes isolated from Taxus canadensis. Tetrahedron Lett. 33, 5173-5176. https://doi.org/10.1016/S0040-4039(00)79125-8
Zamir, L.O., Nedhea, M.E., Zhou, Z.-H., Bélair, S., Caron, G., Sauriol, F., Jacqmain, E., Jean, F.I., Garneau, F.X., Mamer, O., 1995a. Taxus canadensis taxanes: structures and stereochemistry. Can. J. Chem. 73, 655-665. https://doi.org/10.1139/v95-084
Zamir, L.O., Zhou, Z., Caron, G., Nedea, M., Sauriol, F., Mamer, O., 1995b. Isolation of a putative biogenetic taxane precursor from Taxus canadensis needles. J. Chem. Soc. Chem. Commun., 529-530. https://doi.org/10.1039/C39950000529
Zamir, L.O., Zhang, J., Kutterer, K., Sauriol, F., Mamer, O., Khiat, A., Boulanger, Y., 1998. 5-epi-Canadensene and other novel metabolites of Taxus canadensis. Tetrahedron 54, 15845-15860. https://doi.org/10.1016/S0040-4020(98)00994-6
Zamir, L.O., Zhang, J., Wu, J., Sauriol, F., Mamer, O., 1999a. Five novel taxanes from Taxus canadensis. J. Nat. Prod. 62, 1268-1273. https://doi.org/10.1021/np990160s
Zamir, L.O., Zhang, J., Wu, J., Sauriol, F., Mamer, O., 1999b. Novel taxanes from the needles of Taxus canadensis. Tetrahedron 55, 14323-14340. https://doi.org/10.1016/S0040-4020(99)00930-8
Zhang, Z., and Jia, Z. 1990. Taxanes from Taxus yunnanensis. Phytochemistry 29, 3673-3675. https://doi.org/10.1016/0031-9422(90)85303-WZhang, Z., Jia, Z., Zhu, Z., Cui, Y., Cheng, J., Wang, Q., 1990. New Taxanes from Taxus chinensis. Planta Med. 56, 293-294. https://doi.org/10.1055/s-2006-960961
Zhang, Z., and Jia, Z., 1991. Taxanes from Taxus chinensis. Phytochemistry 30, 2345-2348. https://doi.org/10.1016/0031-9422(91)83646-3
Zhang, H., Tadeda, Y., Minami, Y., Yoshida, K., Matsumoto, T., Xiang, W., Mu, O., Sun, H., 1994a. Three new taxanes from the roots of Taxus yunnanensis. Chem. Lett. 23, 957-960. https://doi.org/10.1246/cl.1994.957
Zhang, J.Z., Fang, Q.C., Liang, X.T., He, C.H., 1994b. Title in Chinese. Chin. Chem. Lett. 5, 497.
Zhang, H., Takeda, Y., Matsumoto, T., Minami, Y., Yoshida, K., Wei, X., Qing, M., Handong, S., 1994c. Taxol related diterpenes from the roots of Taxus yunnanensis. Heterocycles 38, 975-980. https://doi.org/10.3987/COM-94-6697
Zhang, S., Lee, C.T.L., Chen, K., Kashiwada, Y., Zhang, D.C., McPhail, A.T., Lee, K.H., 1994d. Structure and stereochemistry of taxuchin A, a new 11(15→1)abeo-taxane type diterpene from Taxus chinensis. J. Chem. Soc. Chem. Commun., 1561-1562. https://doi.org/10.1039/C39940001561
Zhang, S., Lee, C.T.L., Kashiwada, Y., Chen, K., Zhang, D.C., Lee, K.H., 1994e. YunanTaxusin A, a new 11 (15→ 1)-abeo-taxane from Taxus yunnanensis. J. Nat. Prod. 57, 1580-1583. https://doi.org/10.1021/np50113a020
Zhang, Z.P., Wiedenfeld, H., and Röder, E., 1995a. Taxanes from Taxus chinensis. Phytochemistry 38, 667-670. https://doi.org/10.1016/0031-9422(94)00715-6
Zhang, J.Z., Fang, Q., Liang, X.T., Kong, M., He, W., 1995b. Four new taxoids from the needles of Taxus wallichiana Zucc. Chin. Chem. Lett. 6, 971-974.
Zhang, J.Z., Fang, Q.C., Liang, X.T., He, C.H., Kong, M., He, W.Y., Jin, X.L., 1995c. Taxoids from the bark of Taxus wallichiana. Phytochemistry 40, 881-884. https://doi.org/10.1016/0031-9422(95)00370-M
Zhang, H., Sun, H., Takeda, Y., 1995d. Four new taxanes from the roots of Taxus yunnanensis. J. Nat. Prod. 58, 1153-1159. https://doi.org/10.1021/np50122a001
Zhang, H., Tadeda, Y., Sun, H., 1995e. Taxanes from Taxus yunnanensis. Phytochemistry 39, 1147-1151. https://doi.org/10.1016/0031-9422(90)85303-W
Zhang, H., Mu, Q., Xiang, W., Ping, Y., Sun, H., Takeda, Y., 1997. Intramolecular transesterified taxanes from Taxus yunnanensis. Phytochemistry, 44, 911-915. https://doi.org/10.1016/S0031-9422(96)00658-9
Zhang, J., Sauriol, F., Mamer, O., Zamir, L.O., 2000a. New taxanes from the needles of Taxus canadensis. J. Nat. Prod. 63, 929-933. https://doi.org/10.1021/np000053u
Zhang, J., Sauriol, F., Mamer, O., Zamir, L.O., 2000b. Taxoids from the needles of the Canadian yew. Phytochemistry 54, 221-230. https://doi.org/10.1016/S0031-9422(00)00079-0
Zhang, J., Sauriol, F., Mamer, O., You, X.-L., Alaoui-Jamali, M.A., Batist, G., Zamir, L.O., 2001. New taxane analogues from the needles of Taxus canadensis. J. Nat. Prod. 64, 450-455. https://doi.org/10.1021/np0004449
Zhang, M.L., Dong, M., Li, X.N., Li, L.G., Sauriol, F., Huo, C.H., Shi, Q.W., Gu, Y.C., Kiyota, H., Cong, B., 2008a. A new taxane composed of two N-formyl rotamers from Taxus canadensis. Tetrahedron Lett. 49, 3405-3408. https://doi.org/10.1016/j.tetlet.2008.03.115
Zhang, M., Huo, C., Dong, M., Li, L., Sauriol, F., Shi, Q., Gu, Y., Kiyota, H., Cong, B., 2008b. A new pseudo-alkaloid taxane and a new rearranged taxane from the needles of Taxus canadensis. Z. Naturforsch. B 63, 1005-1011. https://doi.org/10.1515/znb-2008-0814
Zhang, M.L., Shi, Q.W., Dong, M., Wang, Y.F., Huo, C.H., Gu, Y.C., Cong, B., Kiyota, H., 2008c. A taxane with a novel 9α, 13α-oxygen bridge from Taxus cuspidata needles. Tetrahedron Letters 49, 1180-1183. https://doi.org/10.1016/j.tetlet.2007.12.045
Zhang, M.L., Dong, M., Huo, C.H., Li, L.G., Sauriol, F., Shi, Q.W., Gu, Y.C., Makabe, H. Kiyota, H., 2008d. Taxpropellane: a novel taxane with an unprecedented polycyclic skeleton from the needles of Taxus canadensis. Eur. J. Org. Chem. 32, 5414-5417. https://doi.org/10.1002/ejoc.200800587
Zhang, M.L., Zhang, J., Dong, M., Feng, S.H., Huo, C.H., Sauriol, F., Shi, Q.W., Gu, Y.C., Kiyota, H., Cong, B., 2009. Two new 11(15→1) abeotaxanes with a 2, 20-epoxy ring from the needles of Taxus canadensis. Z. Naturforsch. C 64, 43-48. https://doi.org/10.1515/znc-2009-1-208
Zhang, M., Lu, X., Zhang, J., Zhang, S., Dong, M., Huo, C., Shi, Q., Gu, Y., Cong, B., 2010. Taxanes from the leaves of Taxus cuspidata. Chem. Nat. Compd. 46, 53-58. https://doi.org/10.1007/s10600-010-9523-7
Zhang, M., Ni, Z., Wang, Y., Dong, M., Li, L., Sauriol, F., Huo, C., Shi, Q., Gu, Y., 2012a. A new taxane from Taxus canadensis needles. Chem. Nat. Compd. 47, 911-913. https://doi.org/10.1007/s10600-012-0102-y
Zhang, K., Li, Y., Ni, Z.-Y., Zhang, M.-L., Wang, Y.-F., Shi, Q.-W., Huo, C.-H., Sauriol, F., Kiyota, H., Gu, Y.-C., Dong, M., 2012b. New 6/8/6-taxanes isolated from the heartwood of Taxus cuspidata. Helv. Chim. Acta 95, 1566-1572. https://doi.org/10.1002/hlca.201200045
Zhang, H.Z., Wang, Y.F., Zhang, M.L., Dong, M., Huo, C.H., Sauriol, F., Shi, Q.W., Gu, Y.C., Wang, H.L., Kiyota, H., 2013. A new alkaloid taxane composed of two N-formyl rotamers from the rooted cuttings of Taxus canadensis. Chem. Nat. Compd. 48, 1035-1038. https://doi.org/10.1007/s10600-013-0458-7
Zhao, Y., Wang, F.S., Peng, L.Y., Li, X.L., Xu, G., Luo, X.X., Lu, Y., Li, L., Zheng, Q.T., Zhao, Q.S., 2006. Taxoids from Taxus chinensis. J. Nat. Prod. 69, 1813-1815. https://doi.org/10.1021/np060345g
Zhong, S.Z., Hua, Z.X., Fan, J.S., 1996. A new taxane diterpene from Taxus yunnanensis. J. Nat. Prod. 59, 603-605. https://doi.org/10.1021/np9600863
Zhou, J.Y., Zhang, P.L., Chen, W.M., Fang, Q.C., 1998. Taxayuntin H and J from Taxus yunnanensis. Phytochemistry 48, 1387-1389. https://doi.org/10.1016/S0031-9422(98)00078-8
Zhou, X.T., Yu, F., Zeng, Q., 2002. Study on the chemical constituents of the heartwood of Taxus yunnanensis. Zhongyao Xinyao Yu Linchuang Yaoli 13, 317.
Zhou, Z.H., Qu, W., Liu, M.Z., Sun, J.B., Wang, M.H., Liang, J.Y., Wu, F.H., 2014. A new taxane diterpenoid from Taxus chinensis var. mairei. Nat. Prod. Res. 28, 530-533. https://doi.org/10.1080/14786419.2014.881360












 a
a b
b






















































































































